Images Collection
View this article in Search Friendly Plain Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
SENSITIVITY TO D-AMPHETAMINE IN SPIDERS AFTER IPRONIAZID
AND IMIPRAMINE
PETER N. WITT, LAWRENCE BRETTSCHNEIDER and ANDREW P. BORIS Department of Pharmacology, State University of New York, Upstate Medical Center, Syracuse, N. Y.
Reprinted from The Journal op Pharmacology and Experimental Therapeutics Vol. 132, No. 2 May, 1981
Copyright © 1961 by The Williams & Wilkins Co.
Printed in U.S.A.
R©printed from Turn Journal op Pharmacology and Experimental Therapeutics Vol. 132, No. 2 May, 1061
Copyright © 1961 by The Williams & Wilkins Co.
Printed in U.S.A.
8529
SENSITIVITY TO D-AMPHETAMINE IN SPIDERS AFTER IPRONIAZID
AND IMIPRAMINE1‘2
PETER N. WITT, LAWRENCE BRETTSCHNEIDER3 and ANDREW P. BORIS3 Department of Pharmacology, State University of New York, Upstate Medical Center, Syracuse, N. Y.
Received for publication October 11, 1960
Iproniazid has been shown to inhibit the catabolism of amphetamine in vitro (Fouts and Brodie, 1956). Using the method for the estimation of ¿¿-amphetamine in biological materials described by Axelrod (1954) it should be possible to measure whether iproniazid also slows down amphetamine destruction in vivo. A decreased catabolism of ¿¿-amphetamine in the body brought about by iproniazid should result in an increased concentration of amphetamine, and an increase in central stimulation should be observed. In previous experiments (Wolff and Hempel, 1951) it has been shown that the effects of different doses of methamphetamine (Pervitin), a drug similar in chemical configuration and effect to ¿¿-amphetamine, can easily be discerned in the web-building behavior of spiders. It was considered reasonable, therefore, to use spiders as a model system for comparing the time course of ¿¿-amphetamine catabolism with and without iproniazid pretreatment with the changes in web-building induced by these drugs.
Iproniazid has also a potent blocking effect on monoamine oxidase (MAO) in vitro as well as in vivo (Zeller et al.} 1952). It is known that the enzyme which catabolizes amphetamine differs from other deaminating enzymes such as MAO with respect to its substrate specificity, intracellular localization, and cofactor requirements (Axelrod, 1959). This suggests that iproniazid blocks more than one enzyme system. H. Niss (personal communication, 1959) determined MAO in spiders which had the same properties as in higher animals. In order to learn more about the two blocking effects, the time course of
1 This investigation was supported in part by a research grant B-1794 from the National Institutes of Health, U. S. Public Health Service.
2 Some of the results have been previously presented (XXI International Congress of Physiological Sciences in Buenos Aires, 1959).
3 Supported in part by a NIH part-time Medical Student Research Fellowship,
the recovery of MAO and changes in the ability to catabolize ¿¿-amphetamine were studied in spiders.
The new drug, imipramine, has shown an antidepressant effect in patients similar to that of iproniazid. Since this drug apparently has almost no effect on MAO activity (E. B. Sigg, personal communication) it seems possible that we have a drug which affects mood without changing the activity of this enzyme system. However, there are no data available substantiating this premise. Therefore, imipramine was given to spiders and the time course of changes in MAO activity was established. One may inquire, too, whether imipramine has no effect on ¿¿-amphetamine catabolism. Measurements of the disappearance of ¿¿-amphetamine from the spider’s body after imipramine medication would answer such a question. Furthermore, comparison of the ¿¿-amphetamine effect on web-building of spiders with and without pretreatment with imipramine would clarify a possible potentiating effect of imipramine on the central nervous effect of amphetamine.
Methods. MAO was determined by measuring colorimetrically the amount of ammonia developed through oxidative deamination of tyra-mine solution, which had been mixed with rabbit liver or spider homogenate, and left for 1 hour at 20°C under continuous oxygenation at pH 7.4. The ammonia produced was compared to that developed by a blank non-oxygenated mixture containing the same ingredients with the exception of tyramine. The recovery was 97.1% of the ammonia liberated, with a standard deviation of ±0.24% (80 measurements). We could detect as little as 3 jug ammonia nitrogen per 100 mg homogenate.4
Tissue samples or whole spiders were weighed, Sorensen’s buffer was added at a ratio of 1:10,
4 Method to be published by Niss, to whom we are grateful for teaching us the procedure.
183
184
Vol. 132
WITT ET AL.
and the mixture was homogenized in a standard way with a Teflon homogenizer. Four ml of a 6.25% cutsoum solution and 1 drop of antifoam were added to each ml of homogenate. One-fourth of a ml of tyramine solution (100 /imol tyramine hydrochloride per ml water) was mixed with 0.75-ml aliquots of homogenate (the amount of tyramine could vary over 50% without changing the results) and oxygen was bubbled through the reaction mixture for 1 hour at 20°C. After 60 minutes the reaction was stopped by the addition of 1 ml of potassium carbonate solution (165 g K2CO3 in 100 ml of water).
For ammonia analysis a modification of the method of Catzias and Greenough (1958) was used. Two ml of 0.036 N sulfuric acid were placed in another test tube, and air was drawn through the sample and acid for 1 hour. By the addition of 5 ml of phenol citrate to the 2 ml of acid, ammonia was transformed into an indole quinone which developed a blue color upon addition of 2 ml of sodium hypochlorite. The color development was completed after 10 minutes at 50°C, and was read in a Klett colorimeter using a 500-570 m¡x filter. Standard curves were constructed with ammonium chloride and were used for the determination of the unknowns.
Accuracy was gained and time saved by performing the whole series of reactions in standardized Klett tubes. Fresh tyramine solutions were prepared twice a week to eliminate correction for nitrogen absorption from the air.
The disappearance of d-amphetamine from the spider’s body was measured by a modification of the methyl orange procedure of Brodie and Udenfriend (1945) as described by Fouts and Brodie (1956). After extraction of d-amphetamine from alkaline spider homogenate into benzene, it was determined as described previously by Axelrod (1954). A standard curve was prepared by running known amounts of amphetamine through the procedure. Blanks of the reagents and homogenates of untreated spiders were used for the zero settings. Each spider weighing between 150 and 250 mg was treated separately. Recovery of amphetamine directly after injection was 96%.
The following drugs were used: iproniazid (Marsilid), imipramine hydrochloride (Tofranil), and dextro amphetamine sulfate (Dexedrine sulfate).6
Each rabbit received 100 mg/kg of iproniazid or imipramine in sterile saline solution by intramuscular injection. On the day of analysis it was killed by a blow to the neck, bled, and about 3 g
6 We wish to thank Hoffmann-La Roche Inc., Geigy Pharmaceuticals, and Smith Kline & French Laboratories, for these drugs.
of liver were removed for the determination of MAO activity.
The spiders were weighed, and the size of a drop of sugar water was determined, which was consumed by each spider within 10 minutes. The concentration of the drug in sugar water was then prepared according to the body weight of each individual so that a spider received 60 or 600 mg ± 10% of the substance per kg of body weight. All drugs were given between 3 and 5 p.m. The drop was attached to the mouth parts of the spider with an injection needle and the animal was observed while drinking. Imipramine apparently had a disagreeable taste for spiders, for frequently they tried to wipe the drops from their mouths. Only those experiments where total ingestion of the sugar drop was ascertained were used in our evaluations. In consideration of the spider’s pre-oral digestion and the per os effectiveness of the drugs when used in higher animals, application by mouth was considered adequate for the webbuilding experiments.
For measuring d-amphetamine in the spiders’ bodies greater accuracy was obtained by injections of iproniazid, imipramine and d-amphetamine. From a microsyringe the drug was given in 1 to 2 microliters of saline solution. It was found that by the use of a fine needle and injection of a small volume of fluid, survival of animals was achieved.
Orb-web building spiders (.Araneus diadematus Clerck, Araneus sericatus Clerck, and Neoscona vertébrala McCook)6 were kept under controlled temperature, humidity, light and feeding conditions (Witt, 1956), in wooden frames, 20 x 20 inches, with sliding glass doors in front and in back, and the webs were destroyed each evening. The next morning the spiders were taken out of the webs built during the previous night. The webs were made visible with Krylon white glossy spray paint and a photograph of each web together with a scale was taken each day on 35-mm Kodak microfile film with a Contaflex IV camera. Special care was taken to have the film parallel to the web. The film was subsequently projected to original web size and measurements were taken.
The changes in webs which represented changes in behavior (Witt, 1952) were evaluated subjectively and objectively. The subjective evaluation was deemed necessary for 2 reasons. (1) The measurements of a few web proportions do not represent the whole web pattern. One portion, namely, the regularity of the spiral, is, for instance, time consuming for routine measurements. (2) Many grossly abnormal webs—e.g.t
6 We are grateful to Dr. Willis J. Gertsch from the American Museum of Natural History for identification of spider species.
1961
AMPHETAMINE IN SPIDERS
185
those built after 600 mg’/kg of d-amphetamine or 60 mg/kg of d-amphetamine 3 days after iproniazid—could not be measured at all and consequently were lost for objective statistical evaluation. The subjective evaluation was carried out in such a manner that 2 experienced persons were shown pictures of control webs and webs built after drugs without knowing which was control and which was experimental. These were graded as “normal” or “abnormal.” Differences in numbers of “normal” and “abnormal” webs in different groups were compared by means of the Chi square test.
To establish the size of a web the photograph was projected in the original size onto white paper and the outline of its outermost spiral thread was followed with a planimeter. The mean of 3 such measurements per web was regarded as sufficiently accurate. The areas of the webs of one spider directly before and after drug treatment were divided one by the other. If webs did not change in size from one day to the next such a quotient was 1. The mean of all quotients in one experimental group was compared to the theoretical value 1. This was based on the assumption that web size does not change in one spider from one day to the next provided no drug was given. Such an assumption was regarded as justified by previous measurements (Peters, 1939; Rieder, 1957) and has been discussed in earlier papers (Witt, 1956).
The angles between radii in a spider’s web are larger at the top than at the bottom, but the change is gradual. Neighboring angles are very nearly the same size, therefore the average difference of neighboring angles all around a web is close to 0. Again it was assumed that the average difference was characteristic for each spider and did not change from one day to the next. On this basis statistical evaluation was carried out as described in the last paragraph.
Results. Effects on MAO. It can be seen from figure 1 that 24 hours after iproniazid, MAO activity was reduced to practically zero in both rabbit livers and spiders. The four values at 5 and 10 days are significantly lower than the controls (P<0.01). After 20 days, MAO activity of rabbit livers was significantly increased over controls (P — 0.0002). The higher standard deviations of the mean values in spiders as compared to rabbits can be attributed to the relatively low activity of the spider homogenate which came close to the lower limit of the sensitivity of our method. We did not establish the part of the body which contained the highest activity.
Imipramine had an effect on MAO activity in rabbits after three applications of 100 mg/kg on 3 days in succession (table 1). The fall of 40%
Fig. 1. Effect of iproniazid on monoamine oxidase activity in rabbit liver and spider homogenate. The scales on the ordinate indicate that a spider homogenate contains about one-tenth of the activity of an equal amount of rabbit liver (190 /tig nitrogen per 100 mg rabbit liver as compared to 19 ng nitrogen per 100 mg spider). Each column represents the mean of between 2 and 20 determinations in between 1 and 11 tissue samples and the standard error of the mean.

WITT ET AL.
Vol 182
186
TABLE 1
Time course of MAO activity after intramuscular imipramine administration to rabbits The average value is followed by the standard error of the mean. The range after 1 day is 203 to 203.4 and after 6 days 111.5 to 133.9. There is a significant decrease in MAO activity after three applications of imipramine on 3 days in succession when the MAO activity was measured on the fourth day as compared to the first day value, and a significant increase 10 days after one application.
| Treatment | MAO Activity in mg N/100 g Rabbit Liver | _No. of | |
| Days after imipramine | No. of applications | for Determinations | |
| No drug | None | 191.4i 3.52 | 7 |
| 1 | 1 | 203.2 | 2 |
| 1 | 3 | 121.5±10.16* | 6 |
| 6 | 1 | 122.7 | 2 |
| 10 | 1 | 299.2i 3.00* | 4 |
| 16-17 | 1 | 216.0Ü9.90 | 4 |
* Significant difference from control.
below normal was significant. The 2 low values after 6 days suggest also a slow inhibitory effect after 1 injection. After 10 days, MAO activity in imipramine-treated rabbits increased signifi-
TABLE 2
Time course of MAO activity in spiders after imipramine application
None of the figures for MAO activity or any of the standard deviations is significantly different from control values at the 1% probability level.
| Treatment | MAO Activity in mg N/100 g Spider | No. of. Determinations | No. of Spiders | |
| Days after imipramine | No. of applications | |||
| No drug | None | 19.2il.63 | 21 | 11 |
| 1 | 1 | 24.li2.44 | 8 | 4 |
| 1 | 3 | 14.4i2.98 | 6 | 3 |
| 5 | 1 | 14.4i3.60 | 4 | 2 |
| 10 | 1 | 14.7il.40 | 7 | 4 |
| 14 | 1 | ll.3il.14 1 | 10 | 5 |
| 20 | 1 | 10.li2.38 | 6 | 3 |
cantly. This effect disappeared again after 16 to 17 days. Measurements of MAO activity after imipramine may lead to equivocal results if the time after drug application is not taken into consideration. Spiders showed no significant change in MAO activity after imipramine under our experimental conditions (table 2). For reasons which are discussed later we may have

196 t
AMPHETAMINE IN SPIDERS
187
TABLE 3
Percent recovery from the spider* s body 17 hours after injection of a single dose of 60 mg/kg of d-amphetamine The spiders had been given one injection of 600 mg/kg of iproniazid or imipramine at different time intervals previously. Each figure represents the mean followed by the standard error. The 2 observations 1 and 2 days after pretreatment represent 2 identical values and the range on the twentieth day was 10 to 14. The control value 17 hours after d-amphetamine alone was 21% ±0.6 (5 observations) .
Compare the relatively quick disappearance of d-amphetamine after imipramine pretreatment with the slow disappearance after iproniazid on the third day (the difference is significant below the 0.1% probability level) and the quick disappearance 20 days after iproniazid.
| Days after Pretreatment | With | % Recovery of d- Amphetamine | No. of Observations |
| 1 | Iproniazid | 90 | 2 |
| 2 | Iproniazid | 69 | 2 |
| 3 | Iproniazid | 68*7 | 8 |
| 5 | Iproniazid | 44*2 | 5 |
| 10 | Iproniazid | 32*0 | 3 |
| 15 | Iproniazid | 22*4 | 4 |
| 20 | Iproniazid | 12 | 2 |
| 3 | Imipramine | 25 | 6 |
missed minor changes. When d-amphetamine instead of tyramine was used as a substrate and all other conditions were kept as described above, our liver or spider homogenates did not show a measurable development of ammonia. This is in agreement with earlier observations (see Axelrod, 1959).
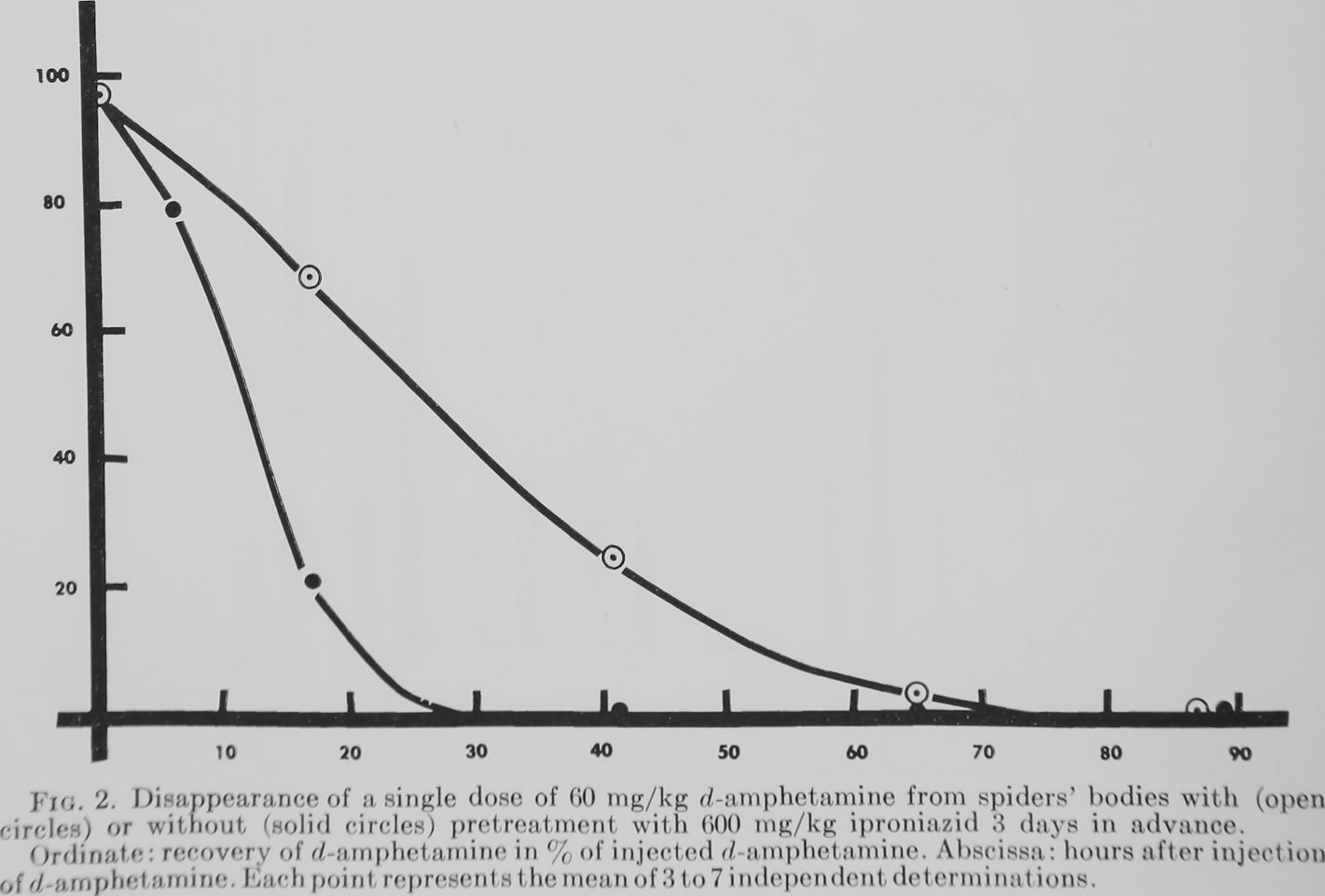
Effects on d-amphetamine disappearance from the body. Immediately after 1 iproniazid injection as well as 1, 2, 3 and 4 days later the methyl orange values in the spider homogenates were not higher than in the untreated control spiders (2 measurements each day). After injection of d-amphetamine the methyl orange procedure yielded high readings which corresponded to a 96% recovery of d-amphetamine. Figure 2 shows that in spiders which were pretreated with iproniazid the disappearance of the substance was significantly slowed down, which we interpreted as an inhibition of d-amphetamine catabolism. The difference between pretreated and non-pretreated animals is particularly striking
in the measurements made 17 hours after d-amphetamine injection. For this reason the 17-hour period—at which 20.8% of the injected d-amphetamine could be recovered from non-pretreated spiders—was chosen for the investigation of the recovery of the spider’s ability to destroy d-amphetamine after one injection of iproniazid. It appears from table 3 that after 15 days the value is equal to that of animals not pretreated with iproniazid. After 20 days the ability to destroy d-amphetamine was increased over control values. Three days after imipramine the catabolism of d-amphetamine appeared not different from controls but significantly different from iproniazid-treated animals.
Effects on web-building. Subjective evaluation: After the large dose as well as after the low dose of d-amphetamine 1 and 3 days after iproniazid, both observers judged a majority of the webs to be grossly abnormal. Examples are shown in figures 3B and 4. The Chi square test indicated a significantly increased number of abnormal webs, below the 1 % probability level, after 600 mg /kg of d-amphetamine as well as after 60 mg /kg in iproniazid-pretreated spiders. Four days after iproniazid, 50% of the d-amphetamine webs were still regarded as grossly abnormal. Eight days after iproniazid 20%, and 10 and 14 days later none of the d-amphetamine webs was considered abnormal. In all groups there was good agreement between the two observers. Neither the d-amphetamine webs after imipramine nor webs after iproniazid alone appeared grossly abnormal.
Objective evaluation: It has been shown previously that methamphetamine affects 3 web proportions (Wolff and Hempel, 1951): the size of the catching area, the regularity of angles, and’ the regularity of the spiral. The last is the most obvious to the eye (see figs. 3B and 4) and the most difficult to measure, and we believe that our subjective evaluation interprets the change in spirals adequately. As expected, the web size was decreased the day after 600 mg/kg of d-amphetamine and 60 mg /kg of d-amphetamine 1 day after iproniazid-pretreatment as well as 2 days after 60 mg/kg of d-amphetamine in spiders treated 3 days previously with 600 mg/kg of iproniazid (P <0.001). Each drug of the above combination, when administered alone in the same dose, was ineffective. The change in size after 4 days was only significant at the 5% level, and after 8 days the figures were back to normal.
188
VoL 132

Fig. 3A. Control web of a spider built on the morning before it received the drug. Note the pathway of the spider out of the web in the upper left corner.
In contrast to these iproniazid effects there was only one group of webs significantly changed after imipramine-d-amphetamine, and the change was in the opposite direction (table 4). Webs were increased in size where 60 mg/kg of d-amphetamine had been given 5 days after imipramine. This change is similar to that described by Wolff and Hempel (1951) after low doses of methamphetamine.
Iproniazid, in a dose which alone did not affect the regularity of angles, caused webs with significantly more irregular angles (P <0.01)— similar to those after the high dose of d-am-phetamine alone (P<0.01)—when followed 3 days later by an ineffective dose of d-amphet-
amine. If d-amphetamine was given 1 day after iproniazid, only a number of webs were found to have highly irregular angles. This accounts for the large standard error of the mean after 1 day (table 5) which is significantly larger than after 3 days (P = 0.03). None of the changes following imipramine application was significant. The average figures for imipramine deviate in the opposite direction from iproniazid (table 5).
Discussion. Certain limitations of our methods have to be taken into consideration if our results are to be interpreted accurately. The MAO activity in liver was 10 times higher than in spider homogenate. The amount of liver used for these measurements was practically unlimited,

Fig. 3B. This web was built by the same spider as 3A 1 day later. The animal had received 600 mg/ kg d-amphetamine 12 hours before web-building time.
Observe the small size of the web, its irregular spiral and angles between radii.
while individual spiders weighed only 36.2 to 227.3 mg with an average of 108.1 mg and were analyzed individually. Consequently many spider MAO figures were not as exact when compared to the rabbit liver results. There is also uncertainty in our assumed linear relationship between body weight and MAO activity in spiders. We established, however, a measurable amount of amine oxidase in each spider independent of species and we were able to show that this enzyme is blocked by iproniazid in spiders as well as in higher animals. There may have been changes in MAO activity even in cases where our figures did not show such a change. Invariably, measurable changes in spiders were similar in quantity and tíme course to the changes in rabbit liver.
Earlier experiments have shown that it is important to time the drug application for spiders so that the peak effect coincides with their
web-building time (Witt, 1956). The distinct delay in the d-amphetamine peak effect 3 days after iproniazid, as manifested in web size and the change of only a few web angles 1 day after iproniazid, show that such a combination has a slower onset of action than d-amphetamine in a high dose alone, i.e., 36 hours as compared to 12 hours. This can well be explained on the basis of the slow absorption of the low dose of d-am-phetamine from the gastrointestinal tract of spiders, which builds up high concentration in body fluids slowly. The low dose becomes effective only because there is probably a minimum of loss through catabolism. Such a change in time of onset of d-amphetamine action may be responsible for the finding that, 4 days after iproniazid followed by d-amphetamine, a change in web size was no longer deteetable in the objective evaluation. The subjective data sug-
WITT ET AL.
Vol 132
100

Fig. 4. A web built 12 hours after application of 60 mg/kg of ¿¿-amphetamine by a spider which had been pretreated with 600 mg/kg of iproniazid 3.5 days previously.
Note the similarity of changes in figures 3B and 4.
gested, however, that there was still a change in the spiral. It is also possible that a little enzyme activity had recovered on the fourth day, sufficient to decrease ¿¿-amphetamine levels in the spider’s body to a less effective level.
The results of ¿¿-amphetamine measurements in homogenates of injected spiders show that iproniazid pretreatment slows down the catabolism of ¿¿-amphetamine. It is likely that what we measured was mainly ¿¿-amphetamine because iproniazid alone did not produce similar changes. If we compare the ¿¿-amphetamine concentration 12 hours after application in the non-pretreated spicier with that after iproniazid it is not surprising that the effects on web-building are different. Only after iproniazid does the low dose of ¿¿-amphetamine produce a high enough drug concentration in the body to cause a change in web-building.
The two doses of ¿¿-amphetamine, 60 and 600 mg/kg, were arbitrarily chosen in a relationship of 1:10. This was based on our assumption that failure to metabolize amphetamine in the spider’s body would increase its effectiveness about tenfold. The results seem to indicate that our assumption was nearly correct. However, the
variation is too large to permit a more exact calculation.
Our figures show coincidence of ¿¿-amphetamine potentiation, changes in ¿¿-amphetamine catabolism, and the decrease in MAO activity. ¿¿-Amphetamine is most effective and most slowly destroyed at the time at which MAO activity is blocked 100%. On the fifth day MAO activity has recovered 40% and the ability of the spider to metabolize ¿¿-amphetamine is restored 65%. On the fifteenth day both values are no longer significantly different from normal. Such coincidence in the time course of changes in enzyme activity with ¿¿-amphetamine catabolism caused by the same drug might suggest that MAO is involved in the metabolism of ¿¿-amphetamine. Axelrod (1955) has shown, however, that “the enzyme which deaminates amphetamine differs from other deaminating enzymes such as amine oxidase” with respect to its substrate specificity, intracellular localization and cofactor requirements. This leads to the possibility that iproniazid blocks more than one enzyme system. The two systems which we have studied are similar enough to recover with about the same time course.
1961
AMPHETAMINE IN SPIDERS
Mil
TABLE 4
Relative web size, control day/day after drug Figures above 1 indicate smaller webs after the drug and vice versa. There is a significant decrease in web size (below the 1% level) in the first and fourth groups and a significant increase in size after imipramine.
| Iproniazid
mg/kg Spider |
Imipramine
mg/kg Spider |
Days Later | ¿-Ampheta
mine mg/kg Spider |
Relative Web Size, Control Day/1 Day after Drug, S.E.M., and Number of Drug Web Measurements | |
| _ | _ | 600 | 2.03±0.41* | 21 | |
| — | n ■ | — | 60 | 1.43±0.25 | 18 |
| 600 | — | . — | 1.16±0.34 | 19 | |
| 600 | • — | 1 | 60 | 1.27d=0.03* | 11 |
| 600 | — | 3 | 60 | 1.51±0.19 | if |
| 600 | — | 4 | 60 | 1.36±0.14 | 6 |
| 600 | HSÜ | 8 | 60 | 1.09±0.27 | 7 |
| 600 | — | 14 | 60 | 1.32±0.19 | 3 |
| — | 600 | 3 | 60 | 0.66±0.27 | 10 |
| — | 600 | 5 | 60 | 0.63±0.19t | 7 |
| — | 1600 | 10 | 60 | 1.04±0.17 | 5 |
| — | 600 | 13 | 60 | 1.59db0.26 | 7 |
* Significant decrease.
t Second day after d-amphetamine 1.67 db 0.18 8 (significant).
t Significant increase.
Imipramine, which we wished to compare with iproniazid, failed to block MAO in a measurable amount in spiders and did not produce significant inhibition of d-amphetamine catabolism on the third day nor did it potentiate d-amphetamine effects on web-building. The 40% decrease of enzyme activity, in rabbit liver following 3 consecutive administrations of this drug and the low activity on the sixth day deserve further investigation. This and the significant rebound effects of imipramine in regard to MAO activity in the liver would indicate that this drug is able to produce partial inhibition of MAO activity under special circumstances.
In following the MAO recovery after a dose of iproniazid, it becomes apparent that there is a statistically significant overshoot of MAO activity around the twentieth day together with an increased ability to catabolize amphetamine. It seems worthwhile to look for a similar phenomenon in man because it might have clinical significance. An increased MAO activity following withdrawal of iproniazid in man may cause symptoms which can be explained by a lack of
TABLE 6
Relative regularity of angles, day after drug/control day
There is a significant increase in irregularity after 600 mg/kg of d-amphetamine alone as well as after 60 mg/kg of d-amphetamine 3 days after iproniazid. There is no other significant change.
| Iproniazid
mg/kg Spider |
Imipramine
mg/kg Spider |
1 Days Later | ¿-Ampheta
mine mg/kg Spider |
Average Relative Regularity of Angles, S.E.M., and Number of Drug Web Measurements | |
| _ | _ | _ | 600 | 1.75±0.11* | 25 |
| — | — | — | 60 | 1.30±0.17 | 21 |
| 600 | — | 1.22±0.23 | 19 | ||
| 600 | — | 1 | 60 | 1.38±0.95f | 12 |
| 600 | _ | 3 | 60 | 1.48±0.06*t | 9 |
| 600 | 4 | 60 | 1.31±0.20 | 6 | |
| 600 | — | 8 | 60 | 1.07±0.09 | 7 |
| 600 | — | 14 | 60 | 1.07±0.17 | 3 |
| 600 | 3 | 60 | 1.15±0.09 | 10 | |
| — | 600 | 5 | 60 | 0.70±0.50 | 7 |
| — | 600 | 10 | 60 | 1.09±0.47 | 5 |
| — | 600 | i 13 | 60 | 1.06±0.35 | 7 |
* Significant increase in irregularity over control (day before drug).
t Significant difference between the 2 standard errors of the mean.
those substances which are now more actively deaminated by the enzyme.
Our measurements did not show any effect of iproniazid or imipramine alone, so that the increase in web size 5 days after imipramine and 1 day after d-amphetamine cannot be explained on this basis. It may be due to the web-enlarging effect of low doses of d-amphetamine (Wolff and Hempel, 1951) or d-amphetamine together with a breakdown product of imipramine.
SUMMARY
Rabbit liver and spider homogenates showed a decrease in monoamine oxidase activity after iproniazid, and recovery of the enzyme in about 15 days. After 20 days, rabbit liver showed a significant increase in MAO activity over controls. At the time of maximal MAO inhibition, the catabolism of d-amphetamine in the spiders’ bodies was slowed down significantly and the effect of d-amphetamine on spiders’ webs had increased about tenfold. On the fifth day after iproniazid the d-amphetaminc sensitivity of spiders was again close to normal and d-am-
WITT ET AL.
Vol 182
192
phetamine catabolism and MAO activity were only slightly slowed down. It is concluded that iproniazid inactivates the system catabolizing d-amphetamine as well as the MAO system.
Imipramine in a single dose showed some effect on MAO activity of rabbit liver or spider homogenate and no effect on d-amphetamine catabolism and sensitivity. Webs were larger when d-amphetamine was given to spiders 5 days after imipramine. Only 3 injections of 100 mg/kg of imipramine brought rabbit liver MAO activity down significantly. There was a significant rebound increase after 10 days. The results indicate that imipramine, unlike iproniazid, has almost no immediate effect on MAO activity. In addition it does not slow down d-amphetamine catabolism and has no detectable d-amphetamine potentiating activity. Web-building of spiders was
apparently unchanged after both antidepressant drugs alone.
REFERENCES Axelrod, J.: This Journal 110: 315, 1954. Axelrod, J.: J. biol. Chem. 214:753,1955. Axelrod, J.: Physiol. Rev. 39: 751, 1959.
Brodie, B. B. and Udenfriend, S.: J. biol. Chem.
158: 705, 1945.
Catzias, G. C. and Greenough, J. J.: Arch.
Biochem. Biophys. 75: 15, 1958.
Fouts, J. R. and Brodie, B. B.: This Journal 116: 480, 1956.
Peters, H. M.: Naturwissenschaften 27:777,1939. Rieder, H. P.: Verh. naturf. Ges. Basel 69: 49, 1957.
Witt, P. N.: Behaviour 4:172,1952.
Witt, P. N.: Die Wirkung von Substanzen auf den Netzbau der Spinne als biologischer Test, Springer-Verlag, Heidelberg, 1956.
Wolff, D. and Hempel, U.: Z. vergl. Physiol.
33: 497, 1951.
Zeller, E. A., Barsky, J., Fouts, J. R., Kirch-heimer, W. F. and Van Orden, L. S.: Experi-entia 8: 349, 1952.