Images Collection
View this article in Search Friendly Plain Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
CHANGES IN FINE STRUCTURE DURING SILK PROTEIN PRODUCTION IN THE AMPULLATE GLAND OF THE SPIDER ARAN EU S SERICATUS
ALLEN L. BELL and DAVID B. PEAKABL
From the Department of Anatomy, University of Colorado Medical Center, Denver, Colorado 80220, and the Department of Pharmacology, Upstate Medical Center, Syracuse, New York 13210
ABSTRACT
The ampullate silk gland of the spider, Araneus sericatus, produces the silk fiber for the scaffolding of the web. The fine structure of the various parts of the gland is described. The distal portion of the duct consist,0 of a tube of epithelial cells which appear to secrete a substance which forms the tunica intima of the duct wall. At the proximal end of the duct there is a region of secretory cells. The epithelium of the sac portion contains five morphologically distinct types of granules. The bulk of the synthesis of silk occurs in the tail of the gland, and in this region only a single type of secretory droplet is seen in the epithelium. Protein synthesis can be stimulated by the injection of 1 mg/kg acetylcholine into the body fluids. 10 min after injection, much of the protein stored in the cytoplasm of the epithelial cells has been secreted into the lumen. 20 min after stimulation, the ergastoplasmic sacs form large whorls in the cytoplasm. Protein, similar in electron-opacity to protein found in the lumen, begins to form in that portion of the cytoplasm which is enclosed by the whorls. The limiting membrane of these droplets is formed by ergastoplasmic membranes which lose their ribosomes. No Golgi material has been found in these cells. Protein appears to be manufactured in the cytoplasm of the tail cells in a form which is ready for secretion.
INTRODUCTION
The ampullate silk gland of the spider Araneus sericatus has as its sole function the rapid production of a single structural protein. This silk fibroin is used for the scaffolding of the web and the dragline trailed by the spider. During the warm part of the year Araneus sericatus build an orb web each day, usually at first light. Web-building takes 20-30 min and uses up most of the silk stored in the silk glands. The amount of protein used amounts to about 10 % of the weight of the glands producing the silk, and rapid synthesis of new protein occurs after the stored protein has been depleted. The events described here are the fine structural
changes that occur following the stimulation of the ampullate gland. Since a great deal of the protein-synthesizing apparatus is devoted to a single protein, this discrete organ is an interesting one for the study of protein synthesis.
Previous work has shown that the gland can be stimulated either cholinergically or by emptying (1). The rate of incorporation of amino acids is greatly increased by stimulation, and this effect can be demonstrated both in vivo and in vitro. Radioautographic studies, in which a pulse of labeled alanine was given at the time of stimulation, show that the first stage is the secretion into
284 Reprinted from The Journal of Cell Biology, 1969, Vol. 42, No. 1, pp. 284-295 Printed in U.S.A.
the lumen of preformed (i.e. unlabeled) protein droplets. This stage is followed by synthesis of new protein, and this material is rapidly secreted into the lumen of the gland. Pretreatment with actino-mycin D or puromycin blocks the synthesis of new protein but does not affect the secretion of the preformed protein into the lumen of the gland (2). Thus, the events following stimulation can be divided into two major parts: secretion of preformed protein and synthesis of new protein.
The gland can be divided into three main sections, on the basis both of gross appearance and of function: the duct, the sac, and the tail. In this study the fine structure of each section is described. The bulk of the synthesis of the silk occurs in the tail of the gland. The fine structural changes in this part of the gland during the stimulation cycle are described and related to previously studied biochemical changes. Previous work on the fine structure of the ampullate gland of spiders appears to be confined to an abstract by Threadgold and Sheldon (3) on Tegenaria derhamii and a preliminary report on Araneus given by the authors at the American Association of Anatomists in 1967 (4).
MATERIALS AND METHODS
Adult female Araneus sericatus were used. For the purpose of descriptive morphology the glands were dissected from the spiders and fixed. Other glands were stimulated by the injection into the body fluids of the abdomen of 1 mg/kg of acetylcholine chloride in 0.||H saline. Control animals were injected with saline alone. Four sets of spiders were examined. In each set the glands were rapidly dissected out at time intervals of 10, 20, 30, and 60 min after stimunBn and wef® fixed in either lR osmium tetroxide in 7.30*8 polyvinylpyrrolidone and 0.25 m sucrose at pH 7.40 or in 3.0% glutaraldehyde in 0.05 m Sorensen phosphate buffer at pH 7.40. The glutaraldehyde-fixed tissues were postfixed in 18 osmium tetroxide in the same, buffer. All tissues were dehydrated in a graded series of ethanols, treated with two changes of propylene oxide, and embedded in Maraglas (5) or Epon (6).
Thin sections were cut on a Porter-Blum MT-2 microtome with a diamond knife, stained with uranyl acetate (7) and lead citrate (8), and examined with an RCA EMU 3F or a Philips EM-300 electron microscope.
Light microscope studies were made by fixing the entire abdomen in buffered 10% formaldehyde and double embedding it in cellulose and paraffin (9) .
Studies of the rate of incorporation of labeled amino acids were carried out by incubating the isolated glands in Krebs bicarbonate buffer. Details of this procedure have been published elsewhere (10).
OBSERVATIONS
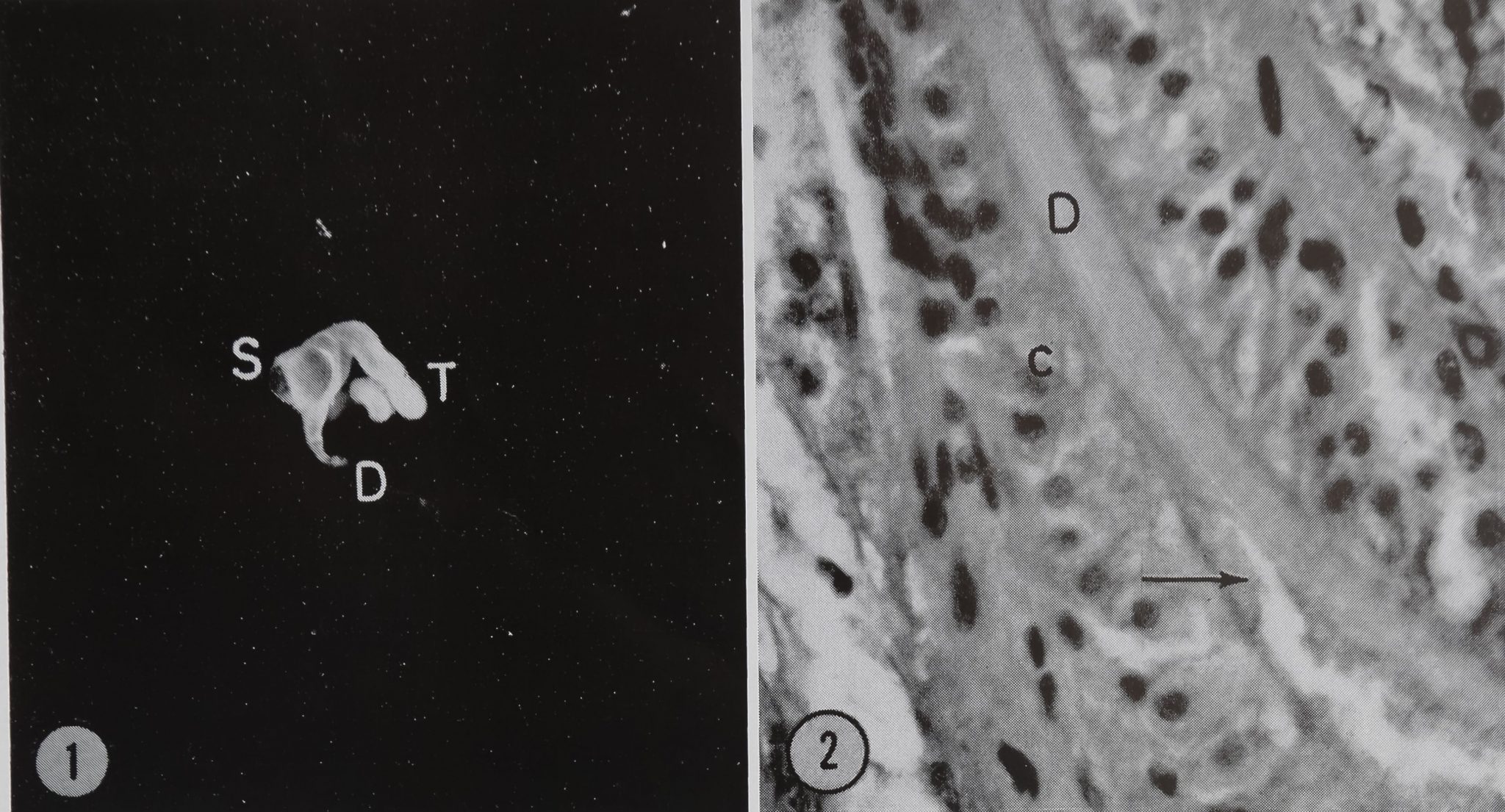
The ampullate silk gland of the spider Araneus sericatus can, from its gross appearance, be divided into three major parts. The long, looped duct (50 /x diameter) leads from the spinneret and joins, via a short, wide region, the wide sac portion of the gland (800 /x diameter). The sac terminates in a long, narrow tail (170 /x diameter) which is coiled around the sac (Fig. 1).
The Duct
A point of interest is that the duct is some five times longer than would be necessary if it had the sole function of joining the sac of the gland to the spinneretHf lie importance of this extra length is unknown, but possibilities would include water removal and/or orientation of the fiber. The ap-BHrance of the duct in the light microscope is Blown in Fig. 2. At this magnification the duH wall appears structureless. Normal cytoplasm and nuclei, apparently associated with the duct wall, are seen.
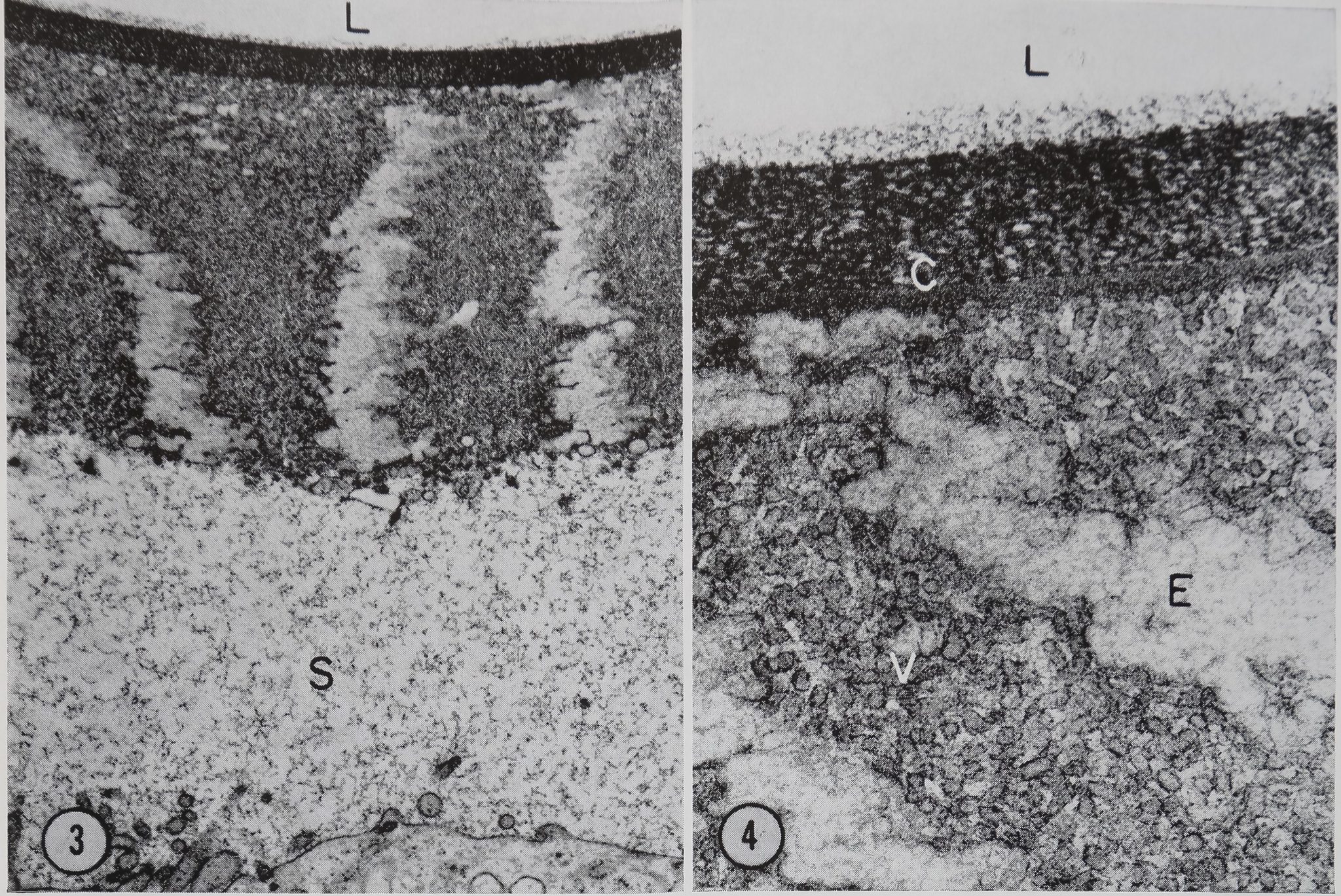
In the electron microscope the duct Wâljli seen to be composed of bothjfejlular and noncellular portidffs. liE^MSlar portion is a single layer of etMraraJSftlls. Tm innermost portion of this layer js^Hi in Fig. 3. ThisRpithelium isReparated from the noncellular tunica intima by a space of 3—4 /x which contains a fine fibroB material (Fig. 3). Although the inner du^ wall Runica intima) appears to be noncellular, it has a remarkably well ordered structure composed of radially oriented bands of tubular or vesiculate structures separated by electron-lucent bands which contain a faint reticulate material (Figs. 3 and 4). The innermost part of the tunica intima is composed of two concentric layers of reticulate material. The outer layer H30 A thick and is|^^B%tron-opaq[ue than the inner layer which is 250 A thick (Fig! 4f§|§j
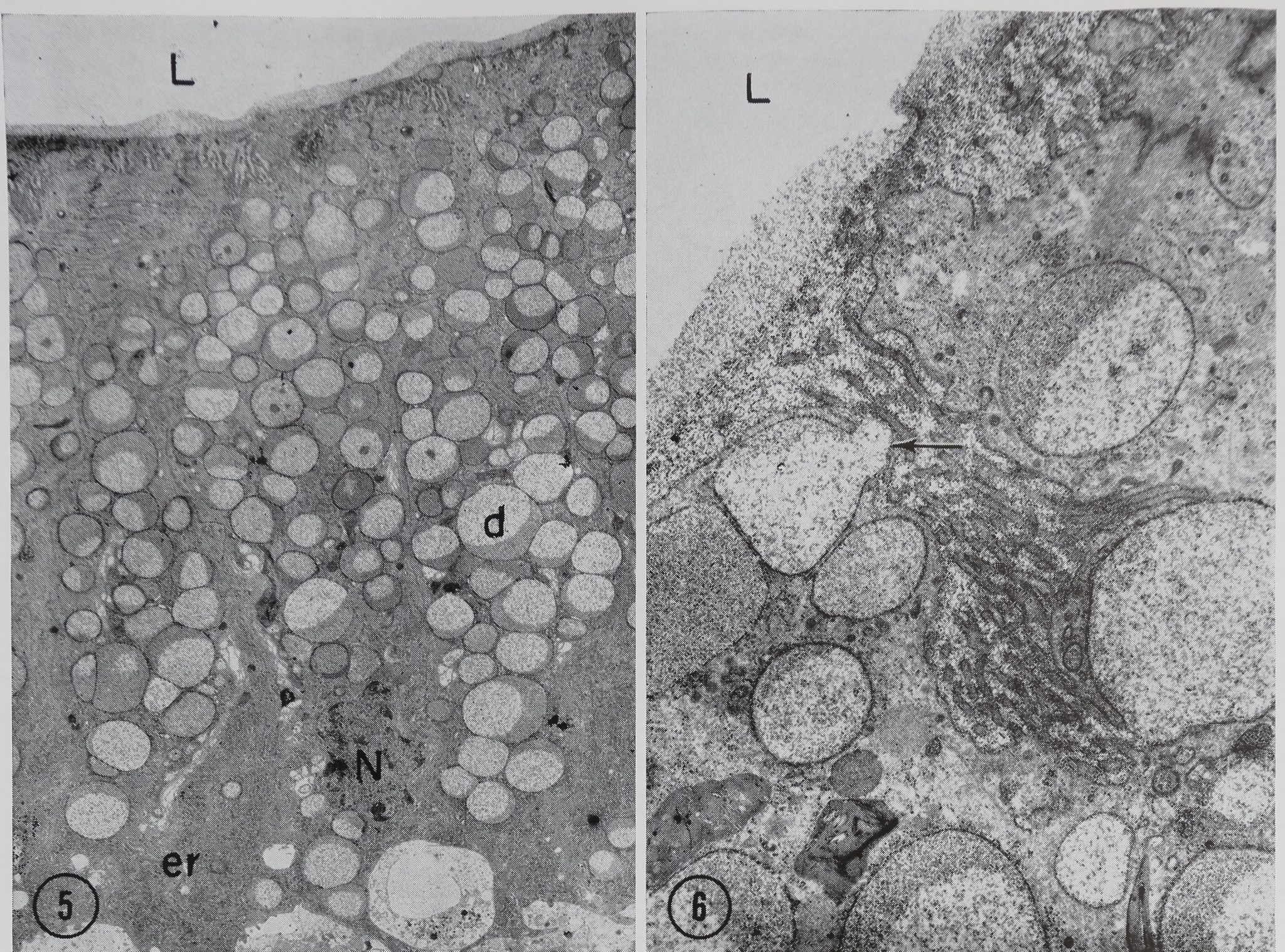
In the transitional zone between the duct and the sac, the wall is composed of columnar epithelial cells 36.0 /jl in height and 9.0 /x in width (Fig. 5). The basal portion of the cells is occupied by a large amount of granular endoplasmic reticulum, mitochondria, and the nucleus. Golgi material is found in the cytoplasm near the apical surface of the nucleus. The rest of the cell (the apical portion) is filled with secretory droplets. The droplets range in size from 1.0 to 3.5 /x and are surrounded by a limiting membrane which varies in thickness from 100 to 250 A. The interior of each droplet contains a material of moderate electron-opacity which has
A. L. Bell and D. B. Peakall Changes in Fine Structure of Spider
285
Figure 1 The ampullate silk spinning gland of Araneus. The coiled tail portion T of the gland is seen on the right. The sac S is swollen by stored silk protein and tapers into the duct D. The cone-shaped tapering portion between the sac and the duct is referred to as the “transition zone” in the text. Approximately X 10.
Figure 2 Light micrograph of a section of the duct of the ampullate silk gland D. In this preparation the whole abdomen was sectioned, thus preserving the duct cells c. Even with this method of preparation, the duct cells often break away from the duct wall as seen here at the arrow. X 565.
a stippled or reticulate appearance. In many of the droplets this material appears to be in two phases of different densities (Figs. 5 and 6). Droplets are frequently seen being secreted into the lumen at the apical surface of the cells (Fig. 6). The plasma membrane at the luminal surface of these cells is modified into numerous pleomorphic microvilli which appear to be surrounded by material similar to that found in the secretory droplets (Fig. 6).
The Sac
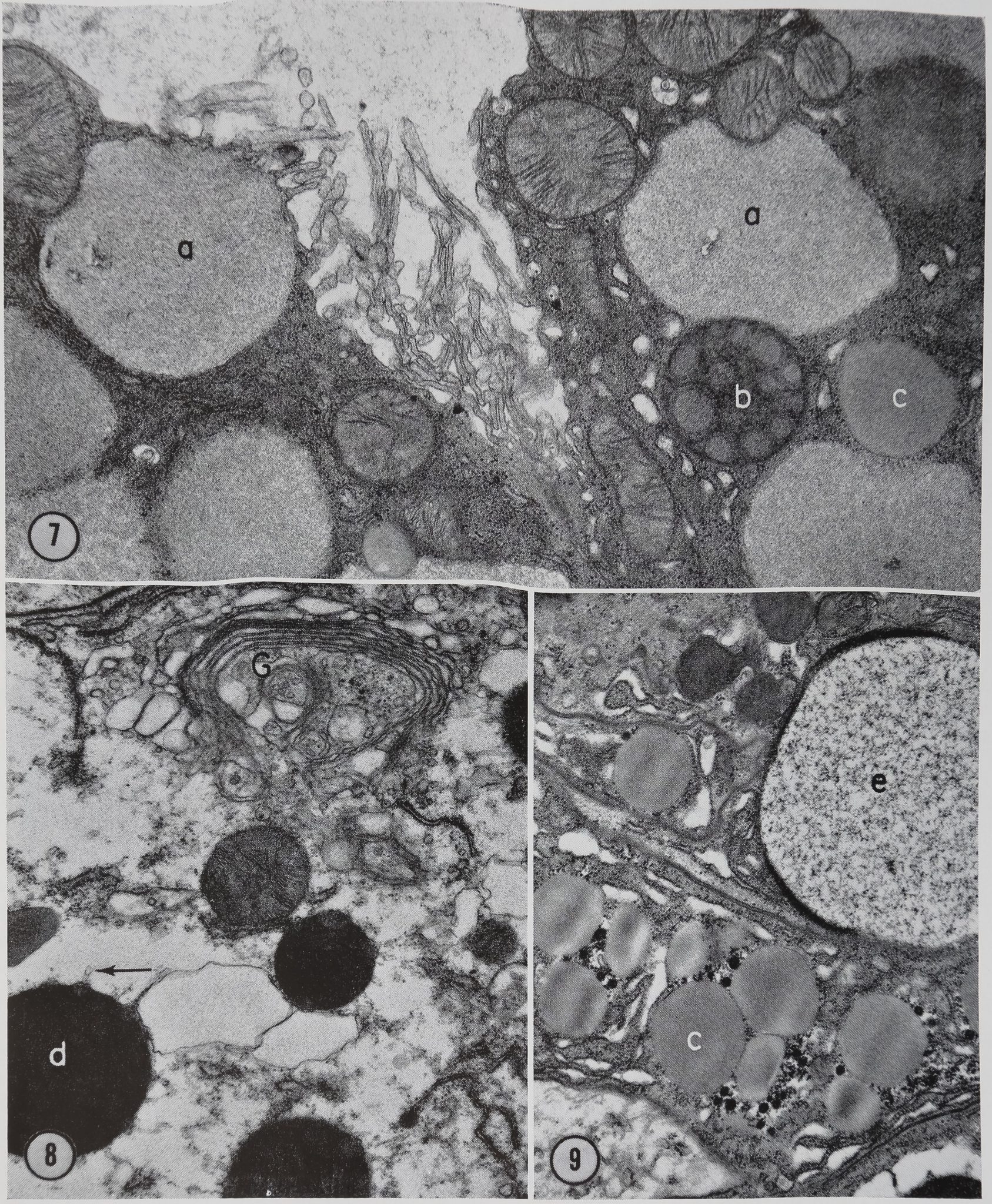
The wall of the sac is composed of a single layer of epithelial cells. There appear to be five types of spherical bodies or droplets in the sac cells which can be differentiated from one another on the basis of their morphology (Figs. 7, 8, and 9). No evidence for the composition or function of these inclusions is available from this study. We are simply describing their morphology as part of the fine structure survey of the ampullate gland. The droplets are designated a through e for descriptive purposes (Figs. 7-9). These letter designations do
not imply any sequence of events but merely serve as a means of identification on the micrographs.
(a) These droplets are identical with those found in the tail of the gland (Figs. 11 and 12) which will be described in the section on that portion of the gland (compare Figs. 7 and 12). ,
(b) This body is composed of homogeneous materials of two different densities (Fig. 7). No definite limiting membrane has been observed surrounding these structures.
(c) Another type of body which is found in the sac is homogeneous and is surrounded by a limiting membrane (Figs. 7 and 9). These structures range in size from 1.0 to 3.0 ji and resemble what have been described by others as lipid droplets.
(d) The fourth type of droplet found in the sac ranges in size from 1.0 to 2.0 [x and has two components. One component is a homogeneous substance of high electron-opacity. The other component is less dense and is found scattered throughout the droplet. This second component is particularly noticeable at the surface of the droplet where it appears as blebs which produce a distention of the
286 Thu Journal of Cell Biology • Volume 42, 1969


Figures 3 and 4, Transverse sections of the distal portion of the duct. A portion of the duct cell is seen at the bottom of Fig. 3. The duct lumen L feat the top in bot’femicrographs. The duct cell is separated from the tunica intima of the duct by a 3^4 i* space S containing a fine fibrillar material. In Fig. 4 the inner portion of the tunica intima is seen at a higher magnification. The vesicular portion V, electron-lucent area E, and double inner component C are visible. Glutaraldehyde fixation. Fig. 3, X 12,200. Fig. 4, X 53,400.
limiting membrane (Fig. 8). Small tubular structures 800 A in diameter are seen extending into the cytoplasm from these blebs (Fig. 8).
(e) This type of droplet is identical with that found in the cells of the transition zone between Hi duct and sac which has been described in pfe” preceding material (compare Figs,. 6 and 9).
The Tail
The epithelium of the tail is composed of columnar cells approximately 60 p high and 15 /x wide. Nuclei are found in the basal portion of the cells. Mitochondria are scattered throughout the cytoplasm. The rest of the cytoplasm is occupied largely by granular endoplasmic reticulum and protein droplets (Fig. 10). No Golgi apparatus has been found in any of the more than 40 tails of glands which have been examined. In the resting gland the protein droplets occupy so much of the cell volume that the nucleus is frequently distorted
(Fig. 10). An occasional membrane-bounded droplet which may be lipid is found Bn close proximity to the protein droplets (Fig. 10).
These cells appear to produce a single type of protein droplet. The droplets range in size from 1 to 8 /x in diameter and contain a homogeneous material of moderate electron-opacity. This material is iJSntical to that found in the lumen (Fig. 12). The appearance of the droplet material is somewhat variable in different experiments even thoughidentical fixation methods were used. However, the material consistently appears more electron-opaque in osmium tetroxide-fixed preparations than in glutaraldehyde-fixed preparations (compare Figs. 11 and 12).
Stimulation Experiments
The main function of the long, coiled tail of the gland has been shown to be the production of over 90% of the silk protein (10). Therefore, the fine
287
A. L. Bell and D. B. Peakall Changes in Fine Structure of Spider
Figures 5 and 6 Transverse section of a cell in the transition zone between the duct and the sac. The nucleus N and the ergastoplasm er are located in the‘base of the cells. Microvilli are seen at the cell surface bordering the lumen L. The rest of the cytoplasm is filled with protein droplets d containing reticulate material of two different electroiSpacities. The arrow points to a droplet being secreted into the lumen. Glu-taraldehyde fixation. Fig. I, X 2,700. Fig. 6, X 12,150.
structural changes following stimulation of silk synthesis have been studied in this region of the gland.
The first observed changes in fine structure are seen 10 min after stimulation. At this point the protein droplets are observed to migrate towards the lumen (Fig. 11). In addition, droplets appear to be emptying their contents into the lumen (Fig. 12).
Between 10 and 20 min afteikstimulation, few protein droplets remain in the epithelium. In addition, a change in the configuration of the granular endoplasmic reticulum (ER) becomes apparent. This change can be seen in Figs. 11 and 13 in which the cisternae of the granular ER are arranged in large whorled configurations. Protein material similar to that found in the lumen appears to be elaborated at the center of the whorls (Fig. 14).
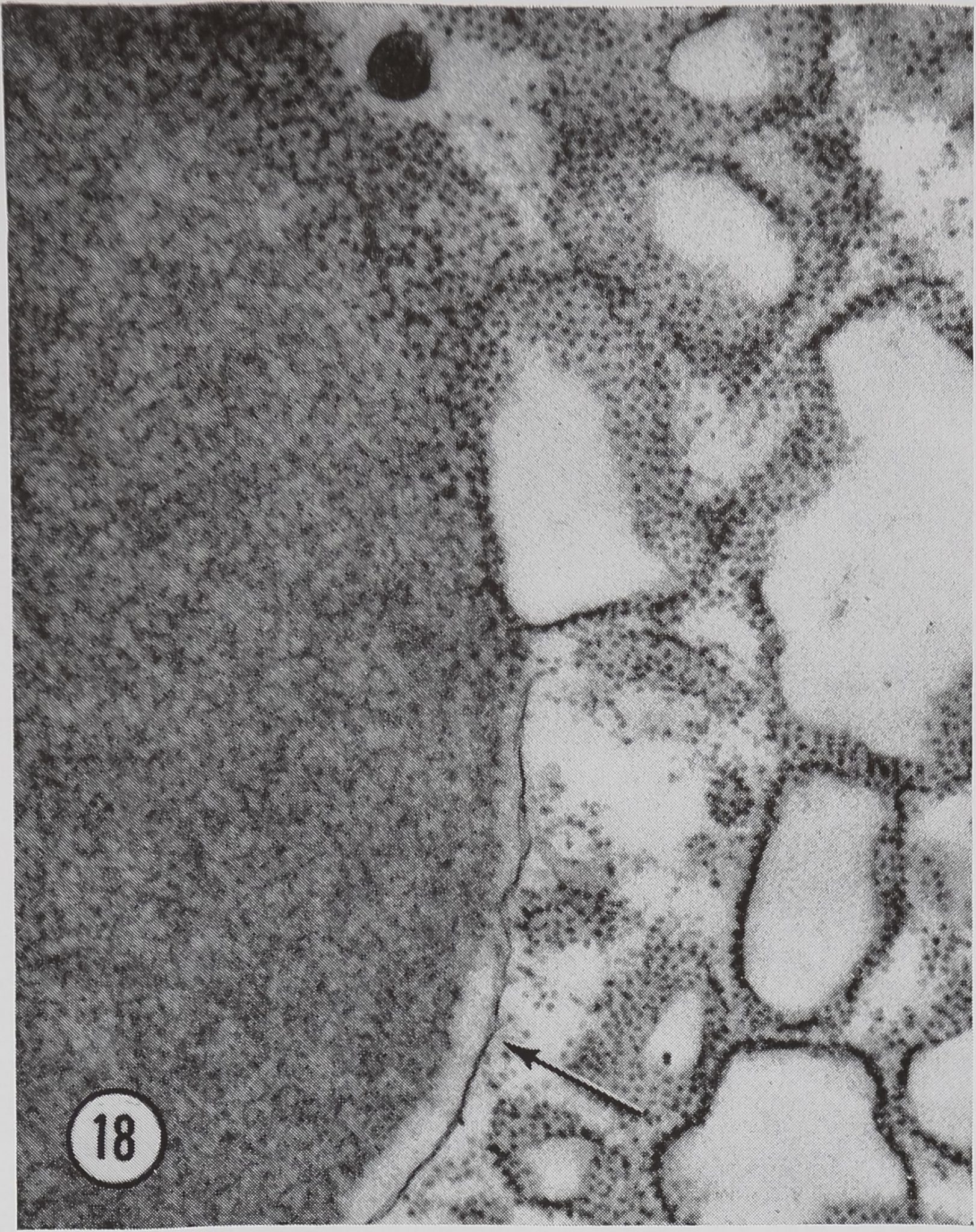
Some ribosomal material appears to be released into the newly formed protein droplets (Figs. 15 and 18)j|This loss of ribosomal material has been confirmed in two ways. First*:spiders fed orotic-6-3H acid show some radioactivity in their silk. Second, light microscope radioautographs of stimulated glands pretreated with orotic-6-3H acid piow light, but above background, labeling in the lumen of the gland. Furthermore, this labeling is not found on sections pretreated with RNase, thus demonstrating the specific nature of the labeling (11). The importance, if any, of traces of ribosomal material in the silk is unknown.
30 min after stimulation, the whorls of rough ER are less evident and the number of new droplets of protein in the cytoplasm have increased. Some of the droplets appear to be in the process of being formed, since connections are still seen be-
288 The Journal of Cell Biology • Volume 42, 1969

Figures 7-9 Electron micrographs of various portions of sac cells. The various types of granules found in the sac cells are labeled a through e and correspond to the descriptions in the text. Note the similarity of types a and e to the granules found in the tail (Fig. 13) and in the proximal duct region (Fig. 6), respectively. The tubular structure extending from the blebs on type d granule is seen at the arrow in Fig. 8. A well developed Golgi apparatus G is also present in Fig. 8. Glutaraldehyde fixation. Fig. 7, X 18,000; Fig. 8, X 16,500; Fig. 9, X 15,200.
A. L. Bell and D. B. Peakall Changes in Fine Structure of Spider 289


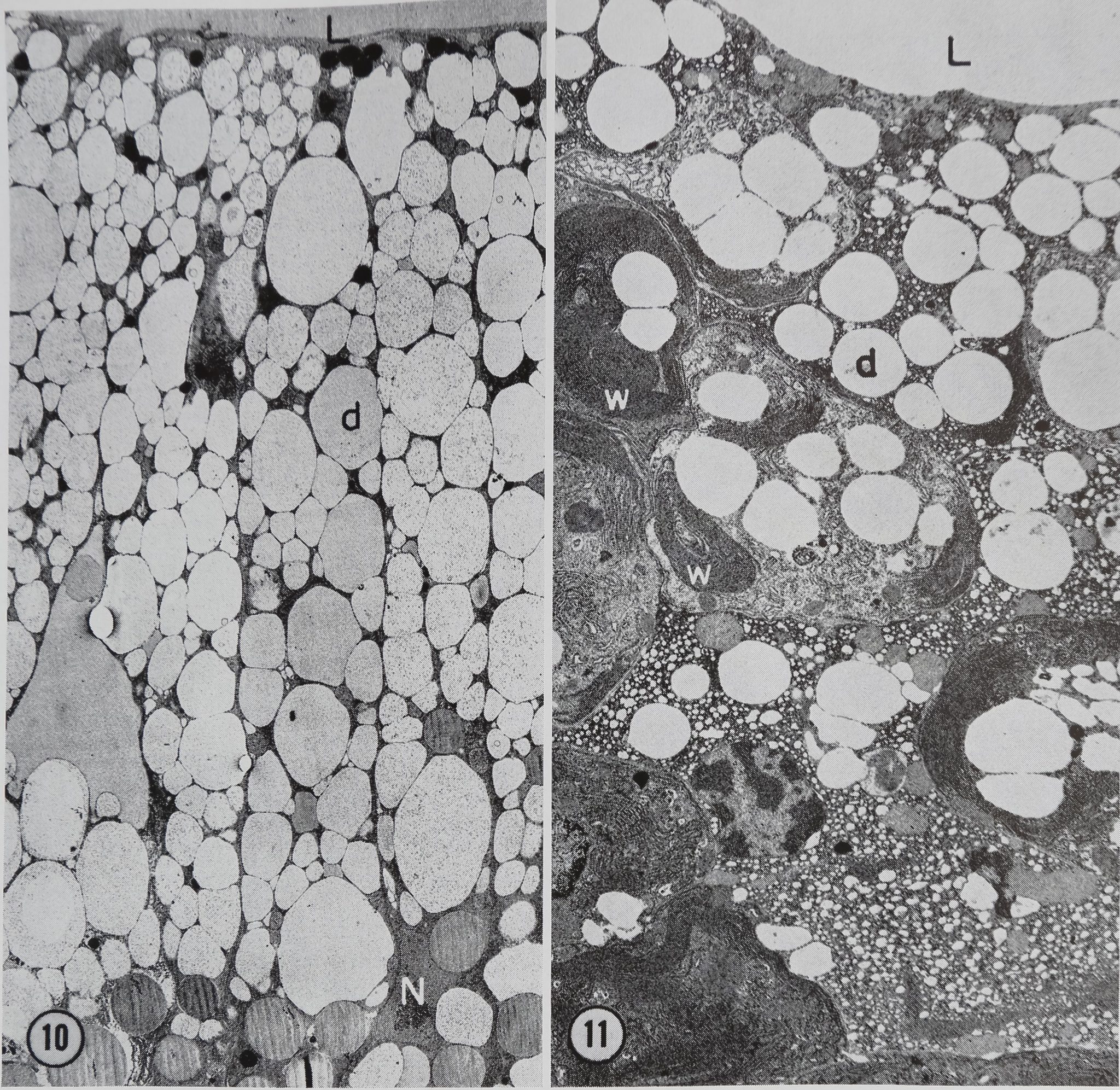
Figure 10 A transverse section through the wall of the tail region of a control (unstimulated) gland. The cells are filled with protein droplets d. A group of small dense bodies ate located near the luminal surface L of the cells. These bodies are presumed to be lipid inclusions. A nucleus N can be seen in the basal portion of one of the cells. Glutaraldehyde fixation. X 2,050.
Figure 11 Wall of the tail region 10 min after stimulation with acetylcholine. Note the decrease in the number of protein droplets d, especially in the basal portion of the gland. Whorls of ergastoplasm w are beginning to form in the cytoplasm. Glutaraldehyde fixation. X 3,600.
tween their surface and the elements of the rough endoplasmic reticulum (Figs. 16, 17, and 18).
60 min after stimulation, the fine structure of the gland cells appears identical to that of the controls.
The changes in fine structure can be correlated with changes seen by light microscope radioautography. The migration of protein in the epithelium can be observed 10 min after stimulation under these conditions. If a pulse of labeled alanine is
given at the time of stimulation, it is observed that f the droplet being secreted is unlabeled. The droplets formed 20 min after stimulation are labeled, and the secretion of labeled material into the lumen continues for 2 hr after stimulation (2). Thus, this procedure shows that active synthesis and secretion occurs over a longer time period than is indicated by the fine structure changes.
During the course of the fine structure work, it
290 The Journal of Cell Biology • Volume 42, 1969

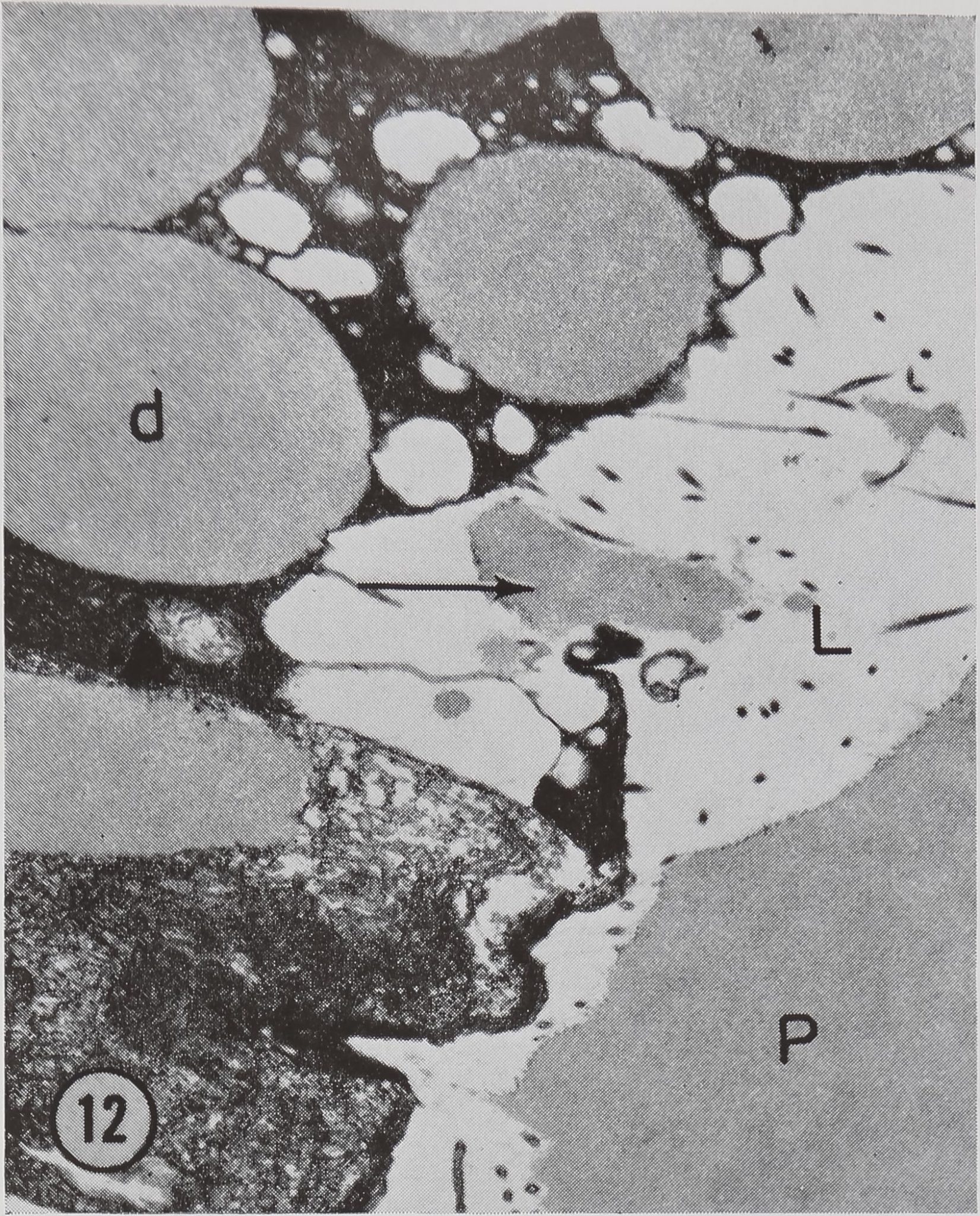
Figure 12 Luminal surface of a cell in the tail region 10 min after stimulation. A protein droplet which appears to be emptying into the lumen L is seen at the arrow. Note the similarity in density of the protein droplets d and the protein in the lumen of the gland P. OsCh fixation. X 7,480.
was noted that in one set of the experiments, which were done in February, glands did not show changes following stimulation. This observation was subsequently correlated with seasonal variation by incorporation studies. Under natural conditions the spiders hibernate during the winter and, although hibernation does not occur in the laboratory, a marked seasonal variation in the activity of the gland does occur. The incorporation of 14C-alanine into protein for the stimulated isolated gland in June as 353 ±31 (10) counts/min per microgram N compared to 59 ± 31 (10) counts/min per microgram N in February. Thus all observations reported here were obtained from experiments carried out during the summer.
sion can be drawn from this study as to the function of the duct, preliminary experiments (11) show that the water content of the silk stored in the sac is higher than that of the silk leaving the duct.
Another function of the duct may be the orientation of the silk into a strong fiber. It is known that the silk protein is in an alpha-conformation in the sac, while in the fiber the protein is in the form of a beta-pleated sheet (13). The exact site of this transformation is unknown, but Wilson (14) considers that the main orientation of the fiber occurs between the spigot and the control valves at the base cf the spinnerets. All these structures are located af the extreme distal end of the duct and have nofl been examined here.
Our fine structure study of the cells of the proximal duct demonstrates that an additional component is added to the silk protein at this point (Fig. 6). Although we do not know what this material is, one possibility would be a polymerase. Braunitzer and Wolff (15) have shown that polymerization of the silk protein does occur. They demonstrated for another orb web-builder, Nephila madagascarfensii, that liquid protein in the sac has a molecular weight of 30,000. After the silk protein passes through the duct and leaves the spider, it has the* form of a fiber with a molecular weight of 200,000-300,000. Similar changes have been found for the silk of the silkworm, Bombyx mori.
The Sac
The sac portion of the gland has been considered by many investigators to be the portion of the! gland concerned with the storage of the protein synthesized by the tail. Experiments on the rate of uptake of labeled alanine show that protein synthesis occurs in this portion of the gland, although the rate is only a quarter of that found in the tail portion (11). Our findings in this study indicate that the epithelium of the sac is complex and that the epithelial cells may represent a transition between the cell type found in the tail and the ceïF type found in the proximal region of the duct.
DISCUSSION
The Duct
The structure of the duct appears to be similar to that found in the silkworm Bombyx mori by Akai (17). The duct is composed of a layer of epithelial cells which appear to secrete the inner noncellular tunica intima (Figs. 2 and 3). Although no conclu-
The Tail
As stated previously, the bulk of the synthesis of the silk protein occurs in the tail portion of the gland. The rate of protein synthesis is very rapid, and the daily production of silk amounts to about 10 % of the weight of the glands when the spider is actively building its web (11). On the basis of morphology, the tail cells appear to be specialized
A. L. Bell and D. B. Peakall Changes in Fine Structure of Spider
291

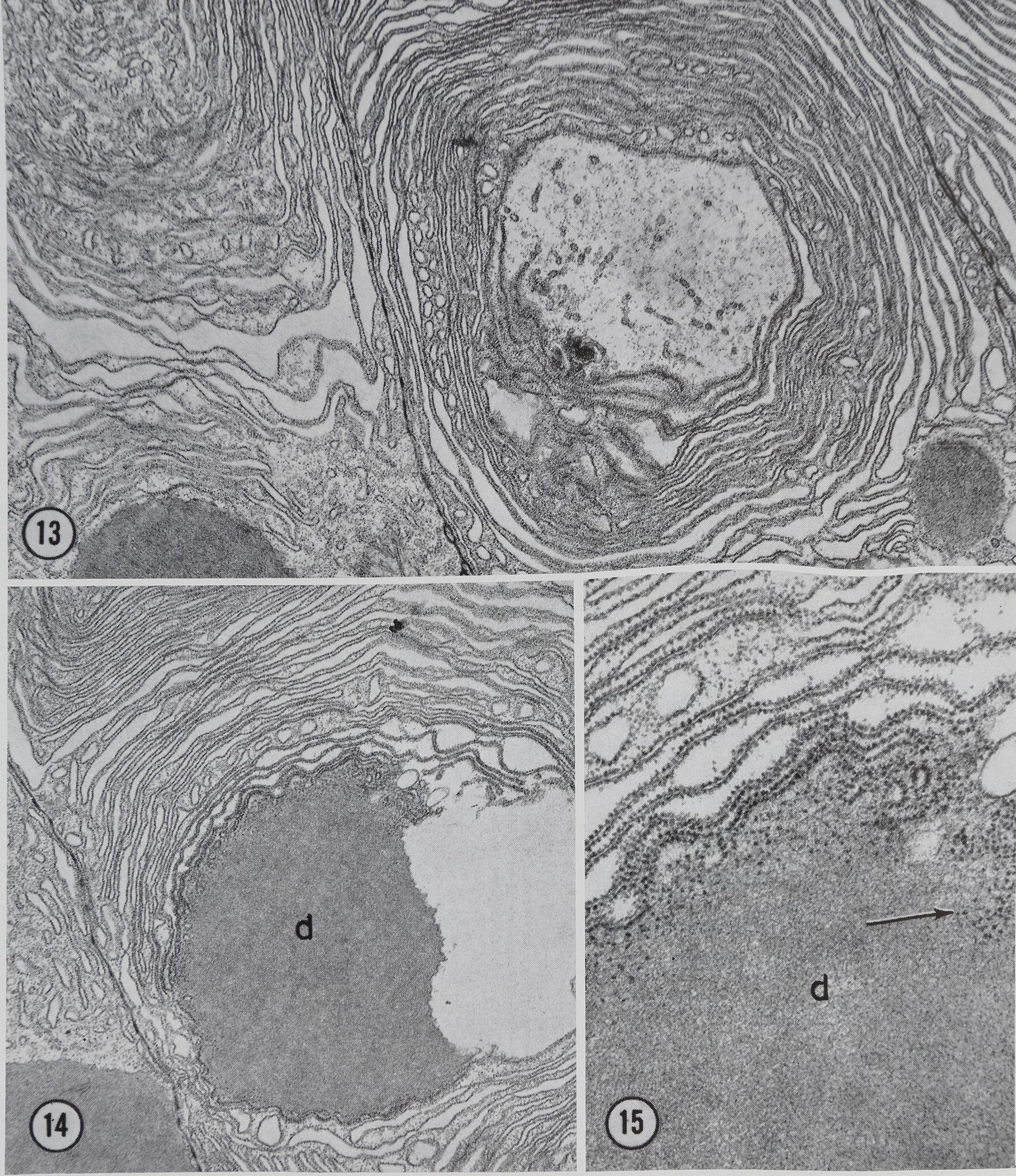
Figures 13-15 Portions of tail cells 20 min after stimulation. In Fig. 13 two whorls of ergastoplasm are visible. Fig. 14 shows an ergastoplasmic whorl in which a protein droplet d is being formed. Fig. 15 is a higher magnification of a portion of Fig. 14 showing ribosomes (arrow) within the developing protein droplet d. Compare the density of the newly forming protein in these micrographs with that of the protein in the lumen of the gland seen in Fig. 12-18 were all fixed in osmium tetroxide. Fig. 13, X 13,750; Fig. 14, X 13,750; Fig. 15, X 42,000.
for the rapid production of large amounts of protein.
In 100’s of tail cells of over 40 glands examined, we have never found any evidence of Golgi ma
terial. In view of the rapid production of large amounts of protein, one would expect that if Golgi material were involved it would be readily detected.
292 The Journal of Cell Biology • Volume 42, 1969
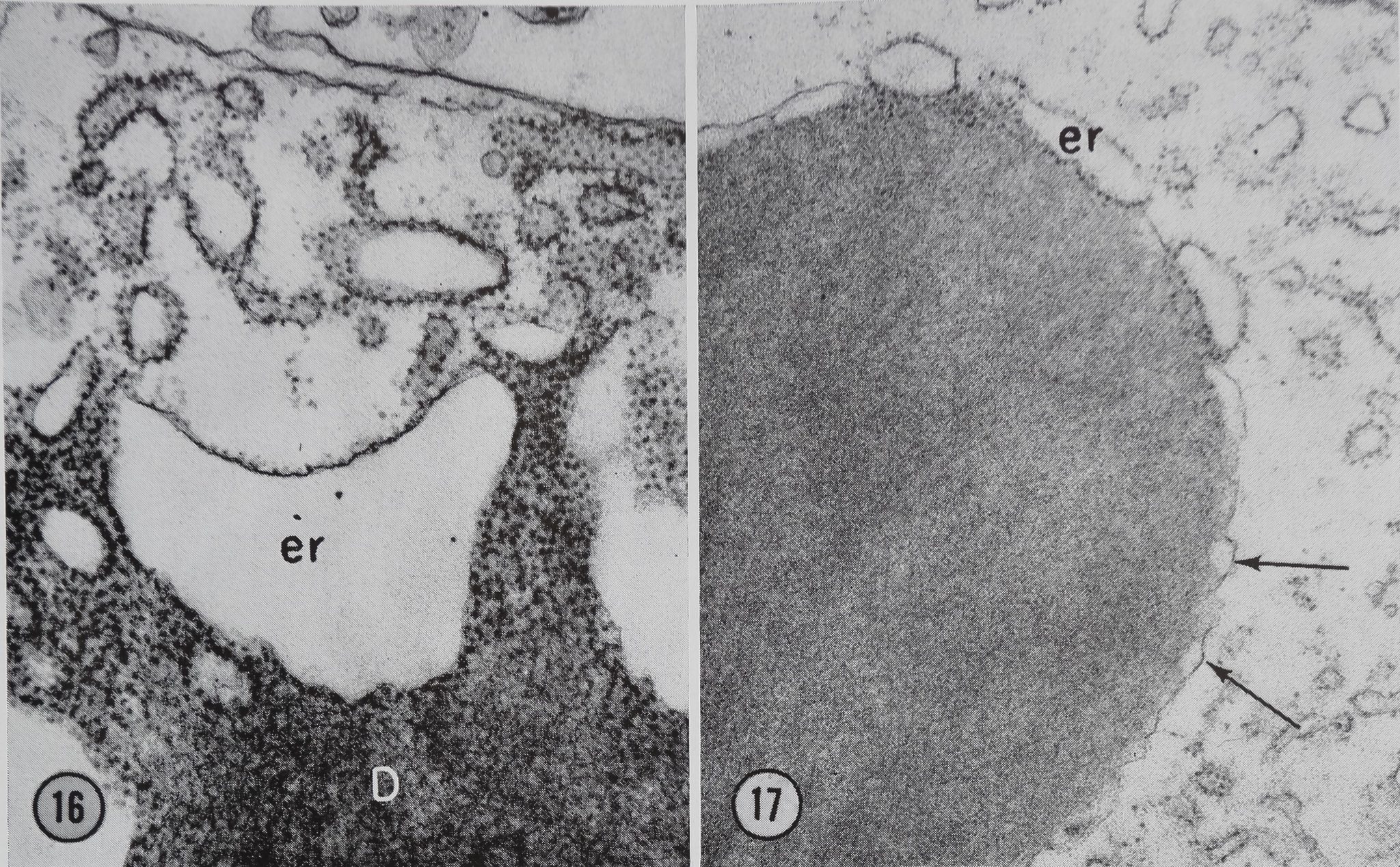
Figure 16 Beginning of m.embran<|#brmation on a completed pr«i;n droplet D. A sac of ergastoplasm er is seen at the surface of the droplet. Most of the ribosofcH have lost from the outer membrane. Borne ribosomes from the inner membrane have been discharged into the protein of the droplet. OsCh fixation. X 42,200.
Figure 17 A droplet in which the membrane is almost complete but is still connected to three ergasto-plasmic sacs er. Note the scalloped appearance of the completed portion of the membrane (arrows). OsCh fixation. X 29,500.
In the sac and duct where protein production proceeds at a much slower rate, Golgi ^material is found in j3R:cell profil^ (Fig. 8). Although one cannot state definitely that none of the tail cells contain Golgi material, we feel that its presence in these cells is highly unlikely.
Thus it appears that the tail cells of the ampul-late gland have developed a somewhat ^Hque method for rapid production of protein. These cells apparently by-pass the concentration andijpack-aging” step in the Golgi apparatus which was first demonstrated by Caro and Palade (16) and which has become known to be a process common to many secretory cells.
Our results suggest that the protein produced in the tail portion of the gland is laid down in a region of cytoplasm limited by whorls of granular ER. This hypothesis would also suggest that the protein may not be transported into the cisternae of the ER. Evidence supporting this concept is available
in Figs. 13-15. ThesSmicrographs Kustrate tail cells 20 Sain a^MStimulation when protein being produced aJ -H rapid rate, ^ yet the .Hlls show no appreciable swelling of ER cisternl and no electron-opaque material within the cisternae. It is also obvious that the elèctron-op acity of the protein in partially completed droplets (Figs, 14 and 15), in droplets which are almost complete (Figs. i|p§18), and in complété dropl||MFig. 12) is identical to the electron-opacity of the protein in the lumen of the tail (Fig. 12) when all these proteins are identically fixed. This observation, coupled with our inability to demonstrate Golgi material in any of the tail cells, suggests that the protein is produced in a form which ■ ready for secretion and undergoes no further concentration.
The formation of the limiting membrane of the droplets appeal!? to involve the loss of ribosomes and of one of the membranes from sacs of ER which are located at the periphery of the protein
A. L. Bell and D. B. Peakall Changes in Fine Structure of Spider 293


Figure 18 High magnification of a droplet at the last stages of membrane formation. Part of the membrane has formed (arrow). Part of the surface of the droplet is still associated with the ER. OsCh fixation. X 33,150.
droplet. Figs. 16-18 showmow this process may occur. These micrographs show glands 30 min after stimulation. At this time sacs of ER are located along the surface of many of the droplets. One of these sacs seen in Fig. 16 appears to be losing ribosomes from both of its surfaces. Figs. 17 and 18 show droplets which have a partially com-
REFERENCES
1. Peakall, D. B. 1964. Effects of cholinergic and
anticholinergic drugs on the synthesis of silk fibroins of spiders. Comp. Biochem. Physiol. 12: 465.
2. Peakall, D. B. 1966. Regulation of protein
production in the silk glands of spiders. Comp. Biochem. Physiol. 19:l||3.
3. Threadgold, J., and H. Sheldon. 1964. On the
fine structure of a new cytoplasmic component in a secreting cell. J. Cell Biol. 23:96A. (Abstr).
4. Bell, A. L., and D. B. Peakall. lj|§7. A unique
mechanism of protein production in the cell of the silk spinning gland of the spider (Araneus sericatus). Anal. Rec. 157:212. (Abstr).
5. Freeman, J. A., and B. O. Spurlock. 1962. A
pie ted limiting membrane. The droplet in Fig. 17 still has at its surface three sacs of ER with a few attached ribosomes, and the sacs are connected to the limiting membrane which has a scalloped appearance. Fig. 18 shows a droplet at the same stage of completion, but at a higher magnification.
It is our hypothesis that the limiting membrane is formed from the membranes of cisternae of granular ER which have lost their ribosomes and have joined together at the surface of the protein droplet.
In contrast with our findings, Threadgold and Sheldon (3) found prominent Golgi material in J what they considered to be the protein-producing part of the ampullate gland of the house spider Tegenaria derhammi. However, their brief account does not specify which portion of the gland was examined. If their studies included parts of the gland other than the tail, then their description of large amounts of Golgi material does not necessarily conflict with our findings. Further, the house spider does not build a new web daily but merely adds threads at intervals to its existing web. Thus the house spider does not have as much need for rapid protein synthesis as an orb web-builder. The elimination of the Golgi material in Araneus may be an adaption for rapid production of silk protein.
This work was supported by grant No. PN-7350 from the National Science Foundation. Part of this work was carried out while Dr. David B. Peakall was an Established Investigator of the American Heart Association.
Received for publication 29 May 1968, and in revised form 14 February 1969.
new epoxy embedment for electron microscopy J. Cell Biol. 13:437.
6. Luft, J. G. 1961. Improvement in epoxy resin
embedding methods. J. Biophys. Biochem. Cytol. 9:409.
7. Watson, M. L. 1958. Staining of tissue sections
for electron microscopy with heavy metals. J. * Biophyt. Biochem. Cytol. 4:475.
8. Reynolds, E. S. 1963. The use of lead citrate at
high pH as an electron-opaque stain in electron * microscopy. J. Cell Biol. 17:208.
9. Steedman, H. F. 1960. Section Gutting in Micros
copy. Charles G Thomas, Publisher, Spring-field, 111.
10. Peakall, D. B. 1965. Regulation of the synthesis
294 The Journal of Cell Biology • Volume 42, 1969
‘
0
of silk fibroins of spiders at the granular level. Comp. Biochem. Physiol. 15:509.
11. Witt, P. N., C. F. Reed, and D. B. Peakall. A
Spider’s Web. Problems in Regulatory Biology. Springer-Verlag OHG., Berlin. In press.
12. Ambrose, E. J., C. H. Bamford, A. Elliott, and
W. E. Hanby. 1951. Water-soluble silk: An alpha-protein. Nature. 167:264.
13. Lenormant, H. 1956. Infrared spectra and struc
ture of the proteins of silk glands. Trans. Faraday Soc. 52:549.
14. Wilson, R. S. 1962. The control of dragline
spinning in the garden spider. Quart. J. Microsc. Sci. 103:557.
15. Braunitzer, G., and D. Wolff. 1955. Ver-
gleichende chemische Untersuchungen fiber die Fibroine von Bombyx mori und Nephila madagascariensis. Z. Naturforsch. 10 b: 404.
16. Caro, L. G., and G. E. Palade. 1964. Protein
synthesis, storage and discharge in the pancreatic exocrine cell : an autoradiographic study. J. Cell Biol. 20:473.
17. Akai, H. 19651? Studies on the ultrastructure of
the silk gland in the silkworm Bombyx mori. IV. Sanshi Shikenjo Hokoku. 19:375.
295
A. L. Bell and D. B. Peakall Changes in Fine Structure of Spider