Images Collection
View this article in Search Friendly Plain Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
Reprinted from The Journal of Morphology
Vol. 132, No. 3, November 1970 © The Wistar Institute Press 1970
Chemosensitive Hairs in Spiders
RAINER F. FOELIX 1
North Carolina Department of Mental Health, Division of Research,
Raleigh, North Carolina
ABSTRACT Spiders possess curved, blunt-tipped hairs on all legs and palps, which differ in many details from the straight, sharp-pointed, tactile hairs: (1) the blunt tip is open to the outside, which can be demonstrated by high resolution microscopy and by the penetration of dyes; (2) the hair shaft has a double lumen which consists of a circular (tube) and a crescent shaped lumen; (3) this hair is innervated by two to three bipolar neurons whose dendrites enter the small tube, where they arborize into 16-20 branches. Multiple innervation and an open tip give strong evidence for a chemoreceptive function. Concluding from their position and distribution on the distal leg parts, a contact chemoreception is tentatively proposed. This interpretation is supported by the close structural analogy to the known contact chemoreceptors in insects. Observation of behavior indicates the importance of a contact chemoreceptor on spider legs. Other possible chemoreceptors in spiders which have been described
previously by other authors are discussed.
Chemoreception in spiders has been clearly established. Numerous investigators from the 18 th century (Homberg, 1707; cit. Blumenthal, ’35) up until recently (Rovner, ’68) have demonstrated behavioral reactions of spiders towards chemical stimuli. Under natural conditions chemoreception is most likely used for testing the quality of food and in recognizing the opposite sex (Bristowe and Locket, ’26; Kaston, ’36). In the experiments which should demonstrate olfaction strong smelling substances such as wintergreen oil or terpineol are generally used (Pritchett, ’04; Blumenthal, ’35; Keller, ’61), and the spiders exhibit a withdrawal or escape re-1 action. The sense of taste is usually tested by offering salt, sugar or quinine solutions (Blumenthal, ’35; Keller, ’62). Most authors conclude that the chemoreceptors are situated on the legs and palps, while some may occur in the mouth region and the esophagus (Millot, ’36, ’46). However, almost every investigator has designated a different organ as the chemoreceptor, and at present it is simply not clear which organs on the spider’s extremities are chemoreceptors.
In other arthropods^ especially in insects, a well-known type of chemoreceptor is the modified hair (Hodgson, ’58; Slifer, ’61; Dethier, ’63; Schneider, ’69) which most often occurs on the antennae and tarsi. Generally, these chemosensitive hairs
are multiply innervated and communicate with the outside. through one or several pores. Because of the small dimensions of the pores, they can only be demonstrated at highest magnification in the light microscope, or, more convincingly, with the electron microscope. The possible occurrence of chemosensitive hairs in spiders was already suggested by McCook (1890) ;
“I have long entertained the opinion that the sense of smell in spiders abides entirely in the delicate hairs . . . ,” but there was never any supporting evidence. A recent study of the tactile hairs of a spider (Foe-lix, ’70) revealed a type of hair which resembles the chemosensitive hairs of insects in most details : this type is the subject of the following discussion.
MATERIAL AND METHODS
Whole mounts of all extremities of Araneus diadematus were examined with the light microscope for distribution and gross-morphology of sense organs. Because of their transparency, exuviae were preferred to normal legs, as they reveal internal cuticular structures. In order to determine the number of sensilla on each extremity, 16 legs and four palps were immersed in xylene and directly counted under the light microscope at magnifications of 100 X and 250 X. For details in the surface structure, a scanning electron
1 Present address : Department of Entomology, North Carolina State University, Raleigh, North Carolina 27607.
J. Morph., 132: 313-334.
313
314
RAINER F. FOELIX
microscope (“Stereoscan,” Cambridge Sci. and Jeol JSM 2) was used.
For histological investigations tarsi of Araneus diadematus or Argiope aurantia were fixed in 5%, phosphate-buffered glu-taraldehyde (Millonig, ’61; Sabatini et al., ’63), post-fixed in 1% 0s04, dehydrated in ethanol, and embedded in Epon or DER 732/332 epoxy resin (Mixture B) over propylene oxide. Whole tarsi as well as isolated bristles were sectioned on a Sorvall MT2 ultra-microtome. For light-microscopy thick sections (2—3 were cut with glass knives and stained with azure B-methylene blue (Richardson et al., ’60). Thin sections were stained with uranyl acetate fin 50% ethanol) and lead tartrate (Pease, ’64) before examining in a Zeiss 9A electron microscope.
Representatives of many other spider families were checked with the light microscope for the occurrence of a chemosensi-tive bristle of the same type.
RESULTS
Araneus diadematus possesses on all palps and legs curved, blunt-tipped hairs, which are arranged serially between the less regular rows of the common tactile hairs. Several morphological features are typical of the curved, blunt-tipped hairs (figs. 1, 3, 4) and allow them to be easily distinguished from the straight, sharp-pointed, tactile hairs: (lÿ A steep angle (50-752) relative to the leg axis; (2) A slight S-shape, ending in a blunt tip; (3) A marked surface striation, which appears as spirals in the light microscope.
Distribution. A careful count of these curved, blunt-tipped hairs on all extremities reveals about 1000. Most occur on the distal leg parts (tarsus, metatarsus, tibia); the density decreases rapidly toward the proximal leg segments. Coxa and trochanter never possess curved, blunt-tipped hairs and the femur only very few on its most distal end. On the tarsus, metatarsus and tibia they are arranged in seven to 8 rows while the fewer hairs on patella and tibia are less regularly distributed. Generally, the curved, blunt-tipped hairs are more abundant on the front side of the leg than on the back side, and again more concentrated on the ventro-lateral part than on the dorsal side (except on the patella).
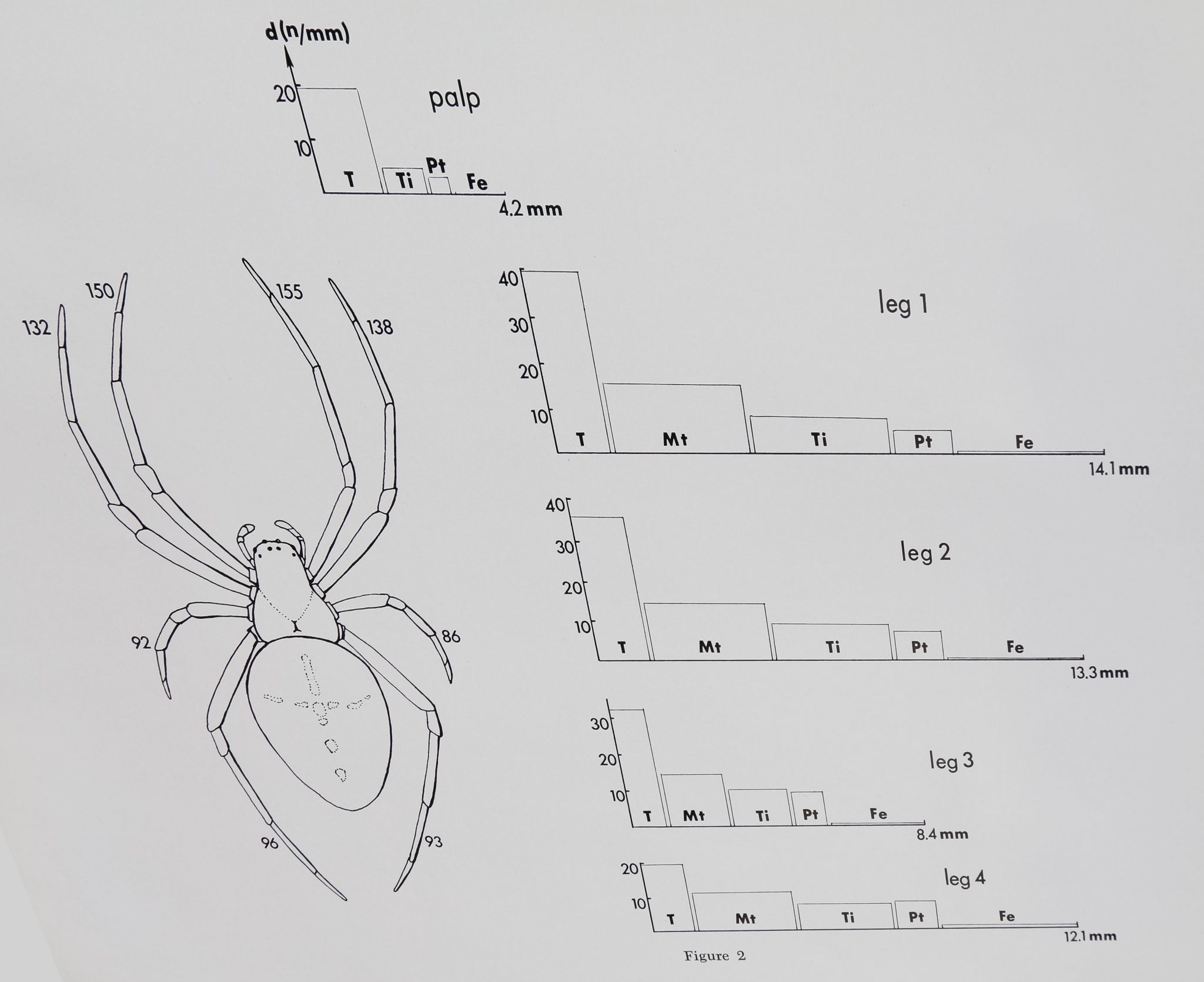
The absolute number of curved, blunt-tipped bristles on each leg as well as the density on different leg parts is given in figure 2. The first two pairs of legs have distinctly more curved hairs than leg 3 and leg 4. Although the number of bristles on tarsus and metatarsus is about the same, the density is much higher on the tarsus since the metatarsus is more than twice as long as the tarsus. A typical count for these blunt-tipped hairs on the different leg parts is given in table 1.
About a dozen curved, blunt-tipped hairs occur on the rim of each maxilla (fig. 5), but none could be found on the chelicerae or the labium. Those hairs on the maxillae show no surface striation but appear comli pletely smoothgfig. 6).
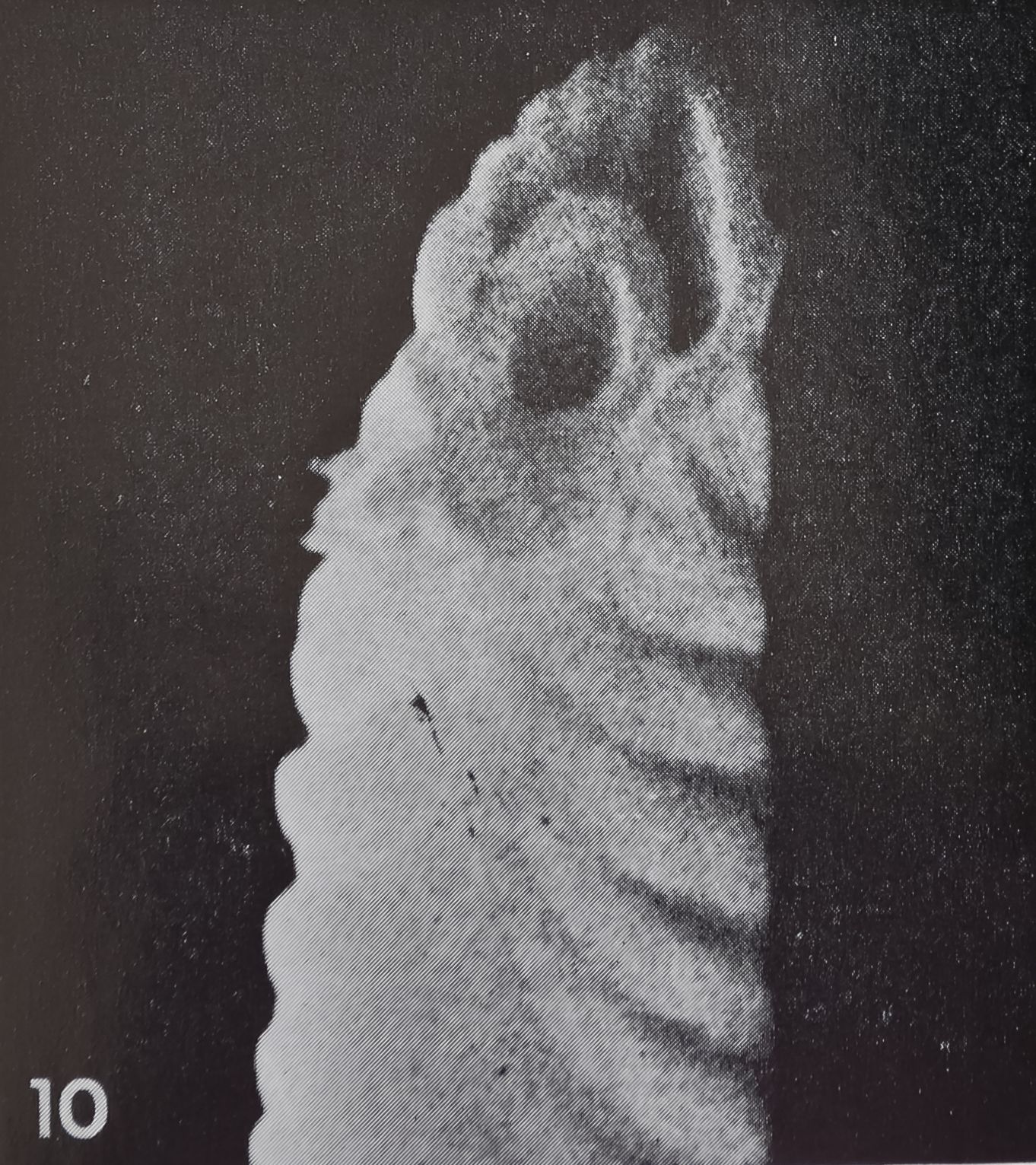
Structure. The curved, blunt-tipped hairs are considerably smaller than the tactile hairs. The length usually varies be-l tween 100-120 ^ (compared to 200 ^ in tactile hairs); the most extreme values measured were 75 ^ and 165 /x, respectively. The diameter at the base is usually 5-6 /x and tapers to less than 2 ^ at the tip. In the light microscope one can observe a fine, oblique striation which seems to spiral around the shaft of the hair. The better resolution of the scanning electron microscope shows that the surface of the hair is smooth toward the outside while fine ribs are only present on the inner side, facing the leg surface (figs. 7, 8, 10). The ribs are rather regularly spaced at intervals of 0.7—0.8 /x.
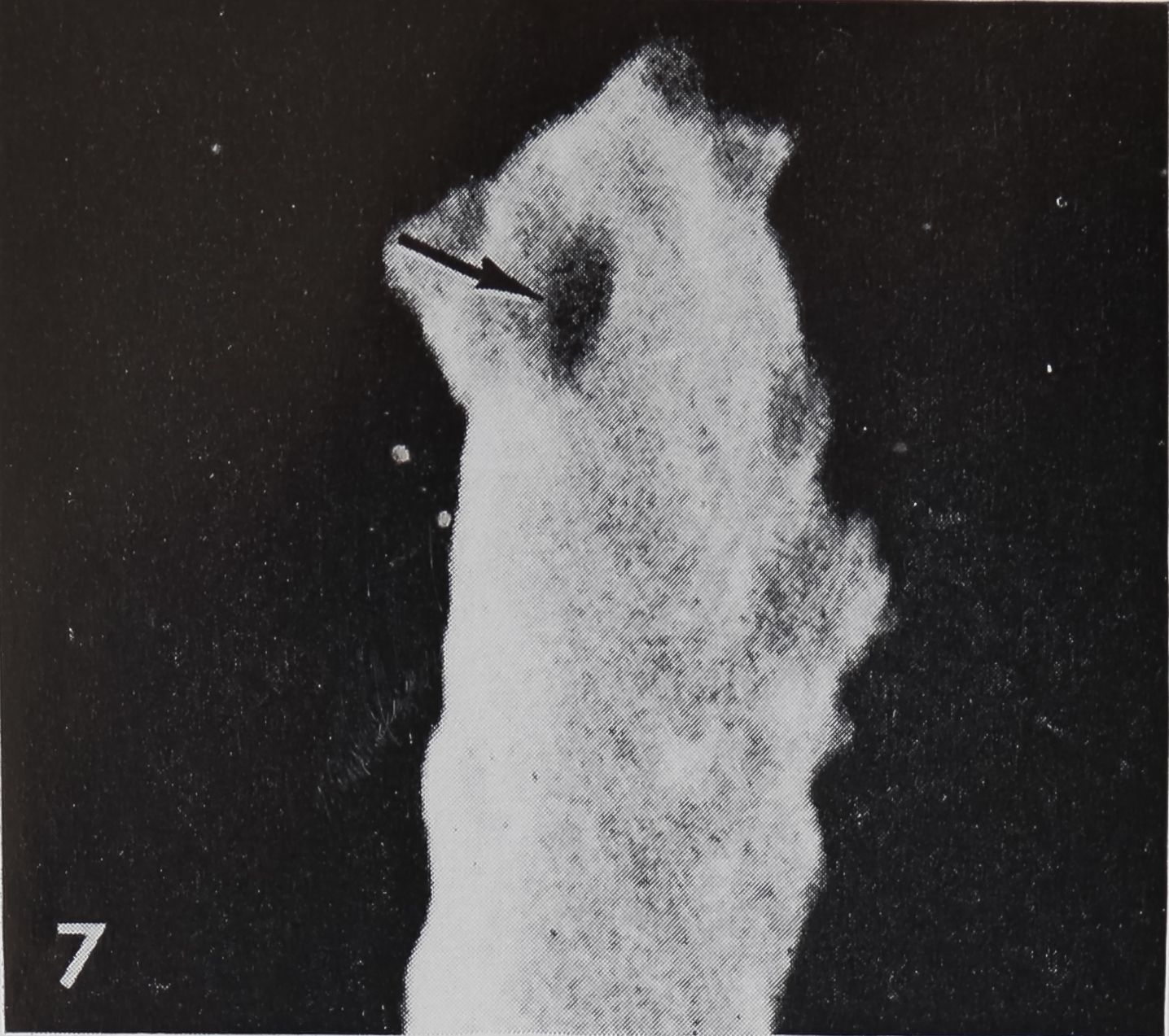
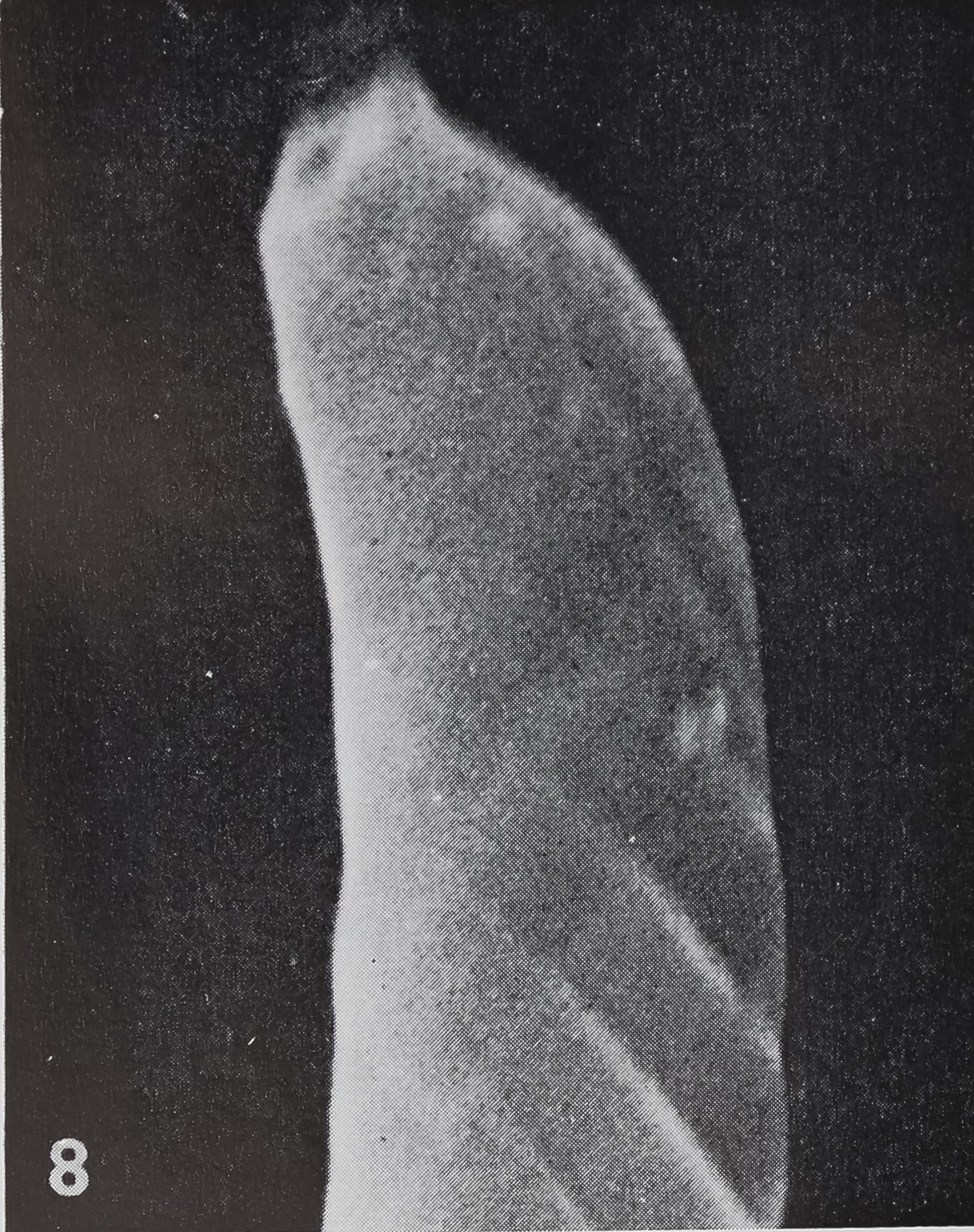
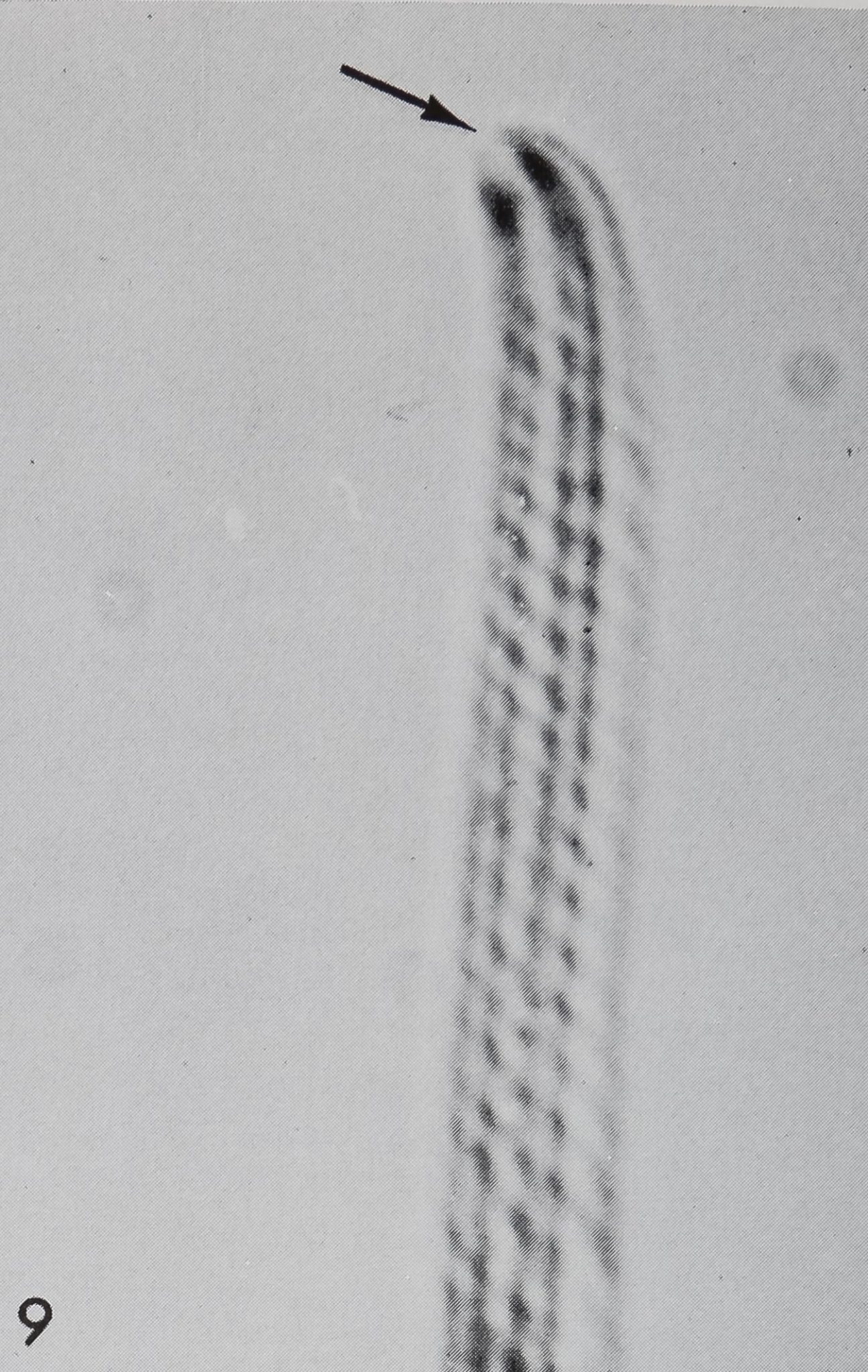
A closer investigation of the blunt tip of the hair reveals a source of communication between the hair lumen and the outside (figs. 7, 8). At the very tip a small area of about 0.5 ^ diameter can be seen as a depression in scanning electron micrographs or as an optically transparent region in the light microscope (fig. 9); This orifice can also be demonstrated by means of crystal violet2 or methylene blue solutions (Slifer, ’60). Those dyes penetrate only at the tip and diffuse downwards into the lumen of the hair, while all other (impermeable) structures remain unstained. Such an open tip is a strong argument for a chemoreceptive function. Therefore, the
2 The author gratefully acknowledges the help of Dr. E. H. Slifer who could localise this type hair by means of the crystal violet technique.
CHEMOSENSITIVE HAIRS IN SPIDERS
315

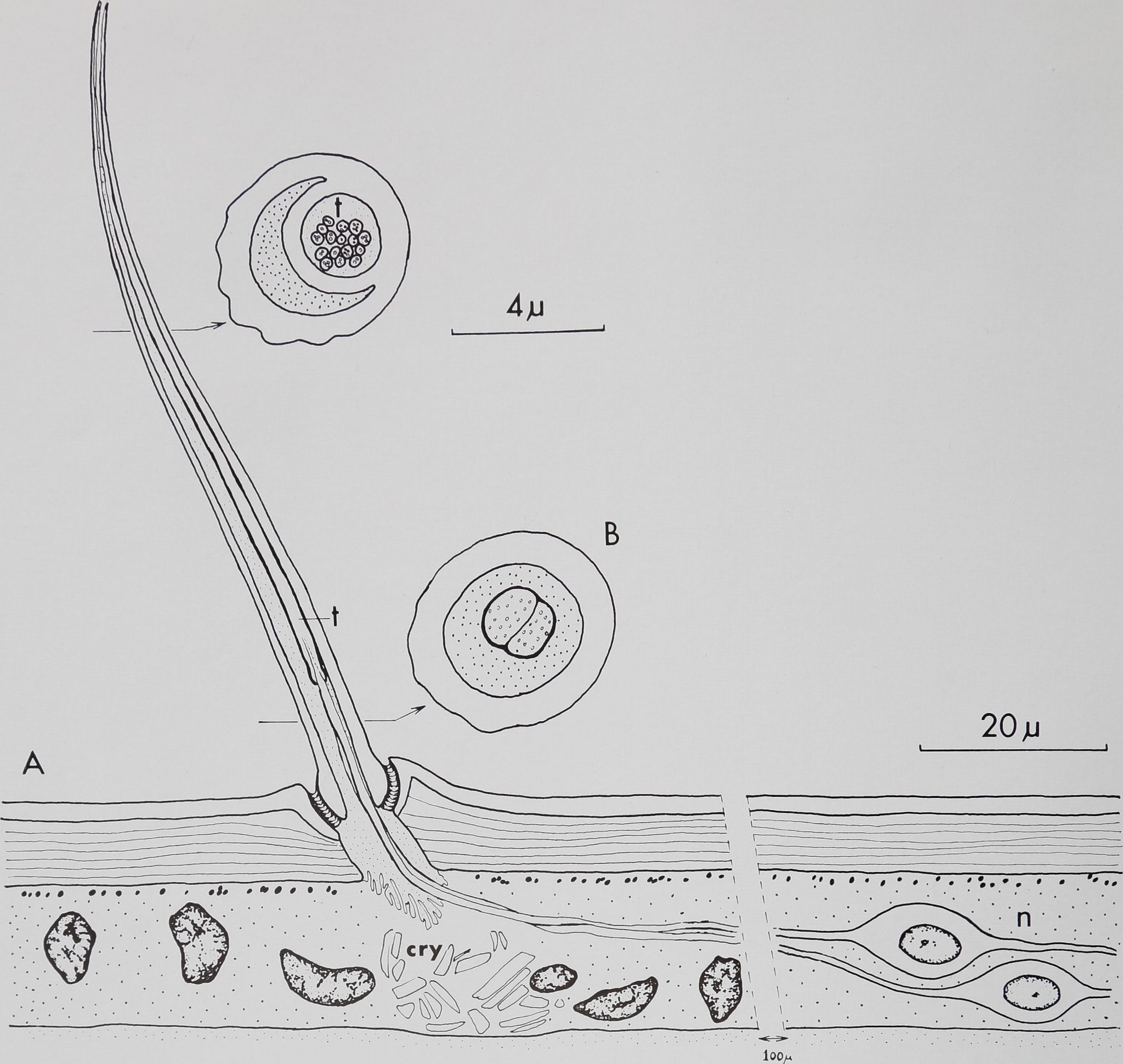
Fig. 1 Diagram of a “chemosensitive” hair. A. Longitudinal section at the level of the light microscope. The curved, blunt-tipped hair inserts at an angle of 50-75° in the leg cuticle (c) Note the double lumen and the open tip. Two or three neurons (n), lying about 100 ^ proximal to the hair, send their dendrites toward the hair base where they enter the lumen. [The spider hypodermis is not syncitial but no clear cell borders are visible under the light microscopeHlB. Cross sections at the level of the electron microscope show 2-3 dendrites enclosed in a cuticular sheath which enter the small, circular lumen (t). These dendrites ramify distally into 16—20 branches, each containing at least one microtubule. Cry, crystalline deposits in the hypodermis.
curved, blunt-tipped hairs will be tentatively referred to as “chemosensitive” in the following discussion.
Another characteristic morphological feature of the “chemosensitive” hairs is their double lumen. When studying exuviae under the light microscope one can clearly see three longitudinal lines composing the shaft of the hair (figs. 6, 9). Scanning electron micrographs of broken chemosensitive hairs show a small circular
lumen of 1-2 p. diameter, surrounded by a crescent-shaped lumen (fig. 10). The circular lumen arises from a cuticular tube which starts about 10 p above the hair base (fig. 11) and runs up to the tip. This tube is always attached to the outer side of the hair shaft, while the crescent shaped lumen always faces the leg surface. The same type of double lumen occurs in the contact chemoreceptors of blow flies (Dethier, ’55; ’63). It has not been found
CO
M
0)
RAINER F. FOELIX

CHEMOSENSITIVE HAIRS IN SPIDERS
317
TABLE 1
| Tarsus | Metatarsus | Tibia | Patella | Femur | |
| Leg 1 | 57 | 57 | 28 | 8 | 3 |
| Leg 2 | 48 | 47 | 27 | 9 | 2 |
| Leg 3 | 34 | 26 | 18 | 9 | 2 |
| Leg 4 | 22 | 34 | 22 | 10 | 3 |
| Palp | 28 | No Metatarsus | 4 | 1 |
Number of “chemosensitive” hairs on the different leg segments of one sub-adult female Araneus diadematus. Note the difference between the first and second leg compared to the third and fourth; this difference was found consistent for several spiders.
in other insects except in some antennal hairs of a beetle larva (Corbière, ’69). The cuticular wall of the “chemosensitive” hair is about 1 n thick. Under the light microscope these hairs therefore appear much lighter than the relatively solid, tactile hairs (wall thickness: 3^).^
Innervation was mainly studied in histological sections stained with methylene blue-azure B. There is no dendritic ending which terminates at the hair base as in the tactile hairs but there are at least two dendrites entering the lumen at the hair base (figs. 12, 14, 15). The corresponding peri-karya may lie more than 100 ^ proximal to the hair base and it is rarely possible to trace the dendrites all the way back to their cell bodies. After entering the extracellular space underlying the hair base (fig. 13), the dendrites follow the outer wall of the hair and together penetrate the small tube which is slightly constricted at its beginning (figs. 14, 15). No further details can be observed with the light microscope. Electron micrographs of cross-sections above the hair base show two or three dendrites which are enclosed by a cuticular sheath. Upon entering the small tube the dendrites split into 16-20 branches, while still enclosed by the cuticular sheath. The dendritic branches contain a varying number of microtubules, the smallest branches being about 600 Â in diameter with only
Fig. 2 Distribution of “chemosensitive” hairs. Small figures in the spider sketch indicate the absolute number of “chemosensitive” hairs found on each extremity of one female Araneus diadematus. Graphs represent density (number/ length) of these sensilla on tarsus (T), metatarsus (Mt), tibia (Ti), patella (Pt) and femur (Fe). Note the high concentration on all distal leg parts, especially on leg 1 and 2.
one central microtubule (diameter about 200 Â). Closer to the tip the cuticular sheath disappears, and the same number of dendritic branches (16-20) is now bathed in a homogeneous fluid (figs. 16, 17). The crescent shaped lumen is also fluid-filled, but appears granular, and may contain some membrane-bound vacuoles. No sections of the very tip were obtained, and thus it is not clear how the dendritic branches finally end and whether they are exposed to the outside at the tip (orifice). Also, due to the considerable length of the dendrites (more than 100 /*), no ciliary structure could be found.
Occurrence. It seemed of interest to find out whether the “chemosensitive” bristles occur only in Araneus or whether they are common in all spiders. First, several other Araneidae (Argiope aurantia, Gasteracantha cancriformis, Micrathena gracilis, Cyclosa conica, Neoscona sp.) were checked and the same type hair could be found. Representatives of other families such as Lycosidae (fig. 18), Salticidae, Oxyopidae, Thomisidae, Agelenidae, Am-aurobiidae, Filistatidae, Ctenidae and Theraphosidae were surveyed; in all cases curved, blunt-tipped hairs were noted. The only variation found were differences in size and surface structure of the hairs (e.g., small spines in lycosids), but all of them showed the characteris tic al slight S-form and the double lumen. In some ground spiders the common hairs are so abundant that one might easily overlook the hidden “chemosensitive” hairs. For instance, in a Filistata sp. (Filistatidae) these small curved hairs were only found after inspection with the scanning electron microscope (figs. 19, 20). In other ground spiders, such as Dugesiella hentzi (Thera-
318
RAINER F. FOELIX
phosidae) or Cupiennus salei (Ctenidae),3 where the legs have almost a fur-like appearance, only the blunt tips can be recognized among the dense scopulae (fig. 21). However, in exuviae the distinct doublelumen is visible, thus confirming their true nature.
Chemosensitive hairs are also already present in young spiderlings (Argiope aurantia) just after leaving the cocoon.
DISCUSSION
The best investigated chemoreceptors in Arthropods are the tarsal and labellar hairs of the blow flies (Phormia and Calliphora). By means of electrophysiological techniques it could be shown that they are contact chemoreceptors, responding to water, salt and sugar solutions (Dethier, ’63; HodgsonH’65, ’68; Wolbarsht and Dethier, ’58; Mellon and Evans, ’61). The morphology and fine structure of these hairs is well known (Grabowski and Dethier, ’54; Dethier, ’55; Larsen, ’62; Peters, ’65; Adams et al., ’65; Wilczek, ’67). They are distinctly curved, blunt-tipped and possess a double lumen. The smaller circular lumen is entered by four dendrites which run up to the open tip. The structural analogy between these contact chemoreceptors of the blow fly and the curved, blunt-tipped hairs in spiders is striking. In fact, the only essential difference is that in the spider hair (Araneus) the dendrites branch inside the small cuticular tubes! whereas they remain straight and unbranched in flies. Dendritic branching inside the hair lumen is, however, found in many chemosensitive hairs in insects (Slifer, ’61, ’68; Slifer et al., ’59; Ernst, ’69); in these casés the cuticle wall is usually perforated by many fine pores instead of one opening at the tip. In contrast to flies, a mechanoreceptor dendrite attached to the hair base, was not noted in Araneus.
One might expect that the open tip is more distinctly visible in the scanning electron microscope than is shown in figures 7 and 8. Some organic material, especially the fluid which fills the hair lumen, might easily clog the orifice at the hair tip and no clearly defined opening would be visible. Stiirckow (’67) describes a sticky viscous fluid filling the tip of the
hair in the blow fly, which can be extruded through the orifice. A protein-containing fluid exuding from the tip of chemorecep-tive sensilla was also found in a grasshopper (Slifer et al., ’57; ’59). In spiders globules were noted adhering to the tip of “chemosensitive” hairs in several cases; it is probable that this material originated from the lumen of the hair tip.
In addition to the close resemblance to contact chemoreceptors in insects there are several other reasons to assume a chemo-receptive function for the curved, blunt-tipped hairs in spiders: (1) The distribution on legs and palps, the highest density being on the most distal part, the tarsus. (2) The position of most hairs. By means of the steep angle of insertion the relatively short curved hairs reach the same level above the leg surface as do the tactile hairs; often they are even more exposed. (3) The behavior of spiders indicates a contact chemoreception. Bristowe (’58) speaks of a “taste-by-touch* sense which is used in the detection of food or the recognition of the other sex. Spiders can differentiate freshly killed flies from old dead flies by tapping the prey with legs and palps; old flies are usually immediately discarded (Eberhard, ’69; Heil, ’36).
Naturally, all these arguments give only indirect evidence for a contact chemorecep-tive function of the curved, blunt-tipped hairs. It is especially difficult in behavioral experiments to rule out all other influencing factors such as mechanical and olfactory stimuli, or the physiological state of the animal (hunger, aggression, etc.). Therefore, an electrophysiological test would be necessary in order to assure contact chemoreception. A brief attempt was made to stimulate and record from the curved, blunt-tipped hairs in Araneus with water, salt and sugar solution or “fly-juice,” with the same capillary-technique as applied in the blow fly (Hodgson et al., ’55; Hodgson and Roeder, ’56). No responses have so far been obtained, but this may be due to technical deficiencies. A more extensive study might elucidate the nature of stimulating substances as well as the threshold concentration.
3 Exuviae of these spiders were kindly supplied by Dr. Rathmayer (Konstanz) and Dr. Barth (Munich).
CHEMOSENSITIVE HAIRS IN SPIDERS
319
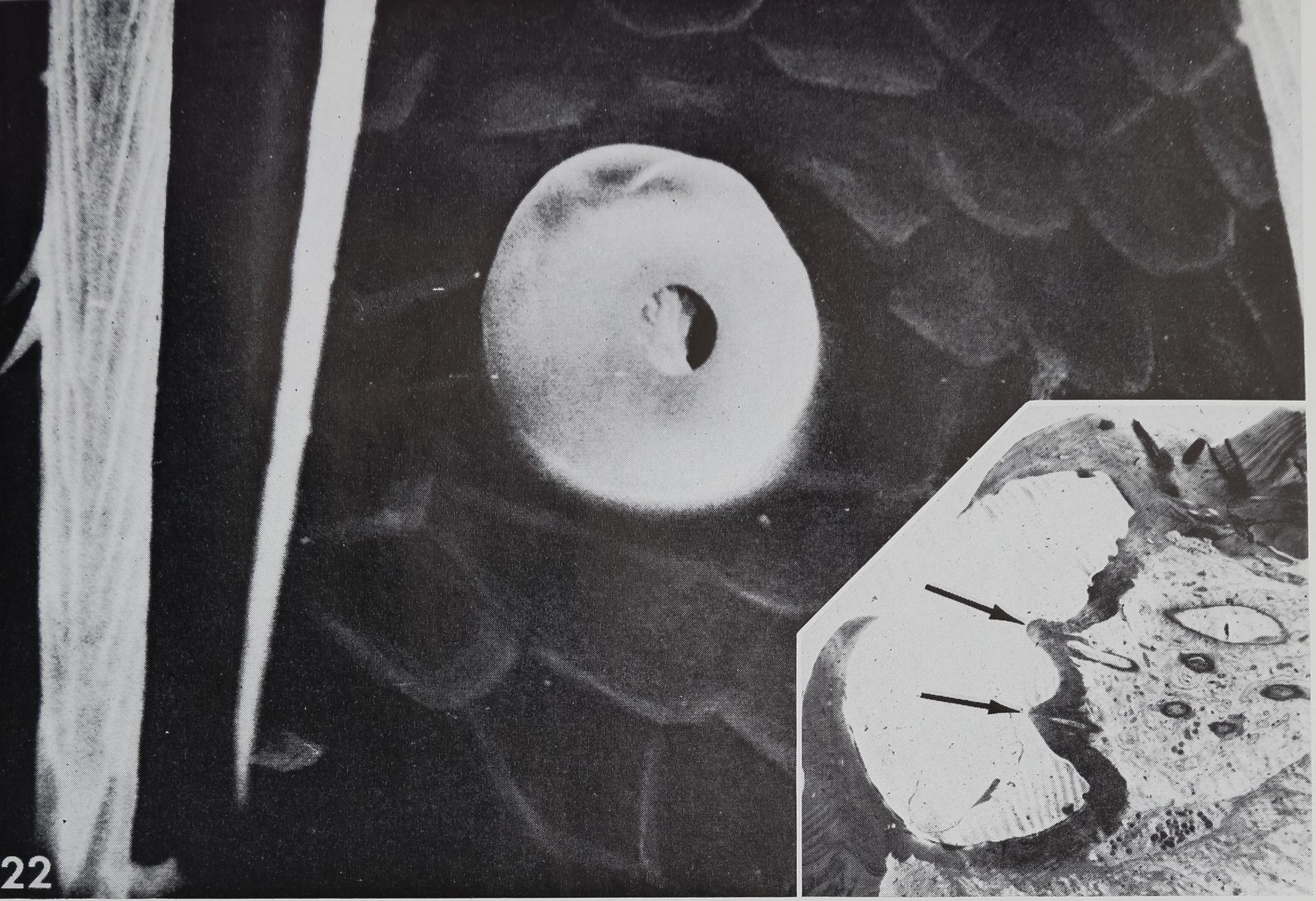
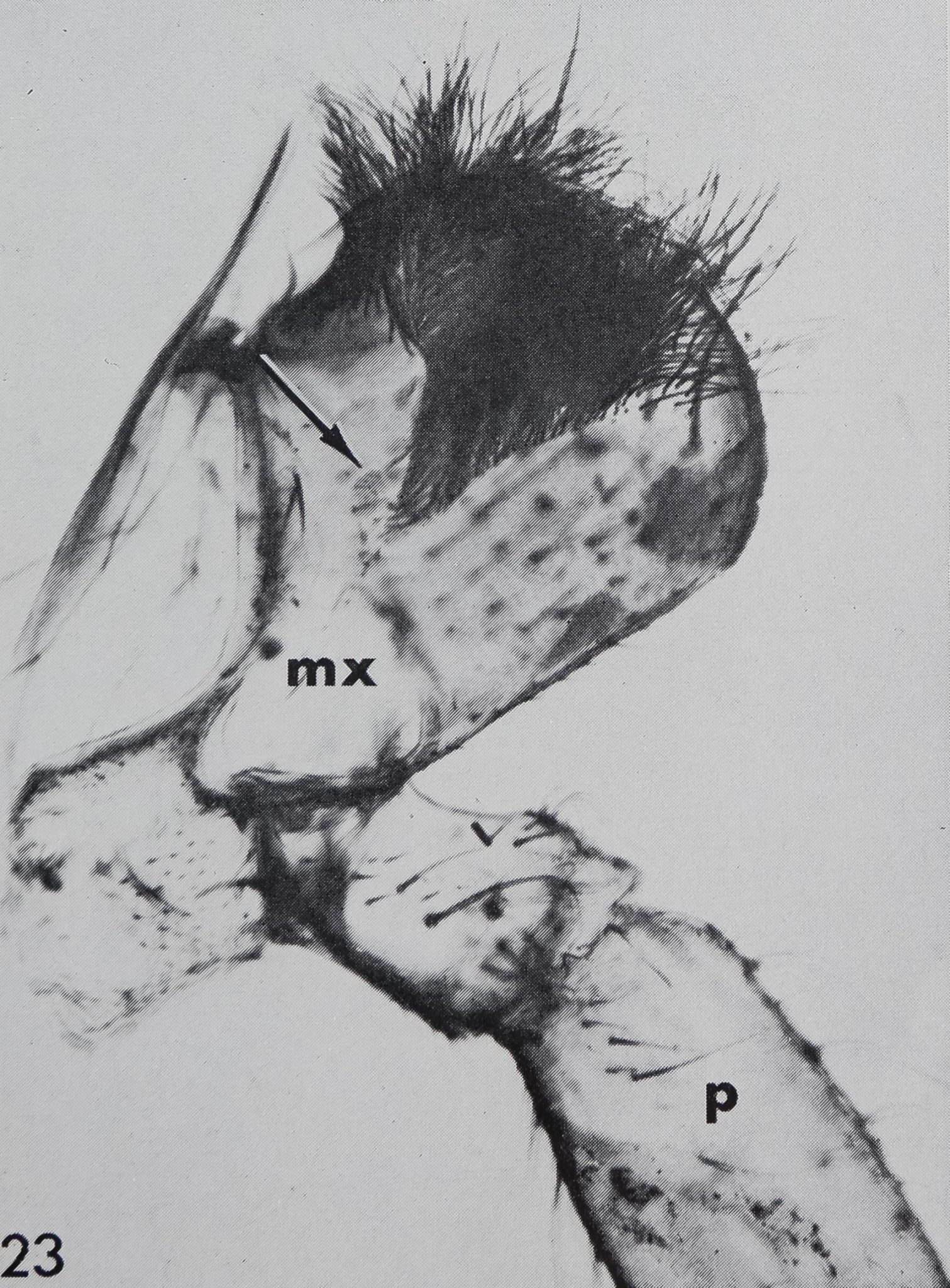
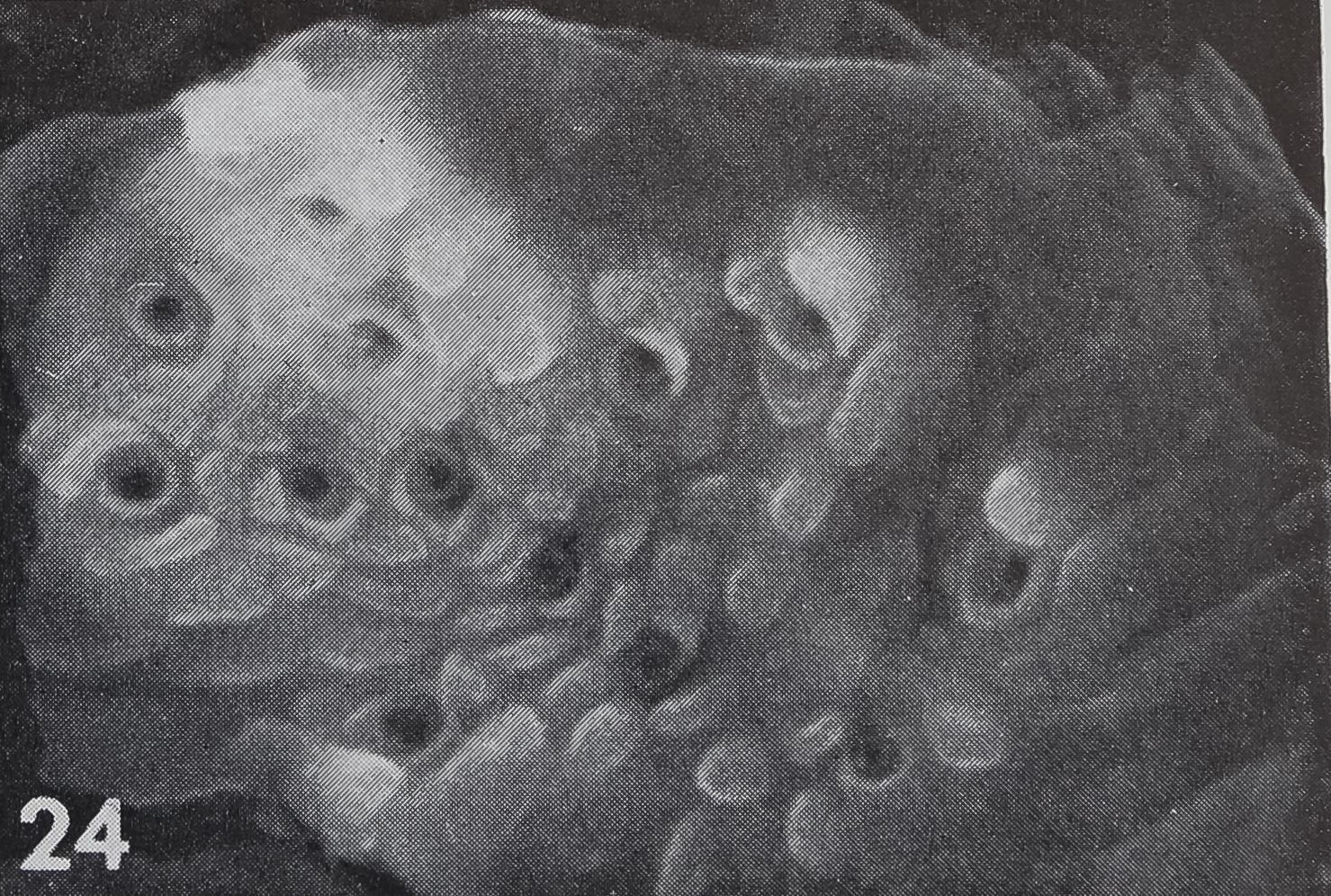
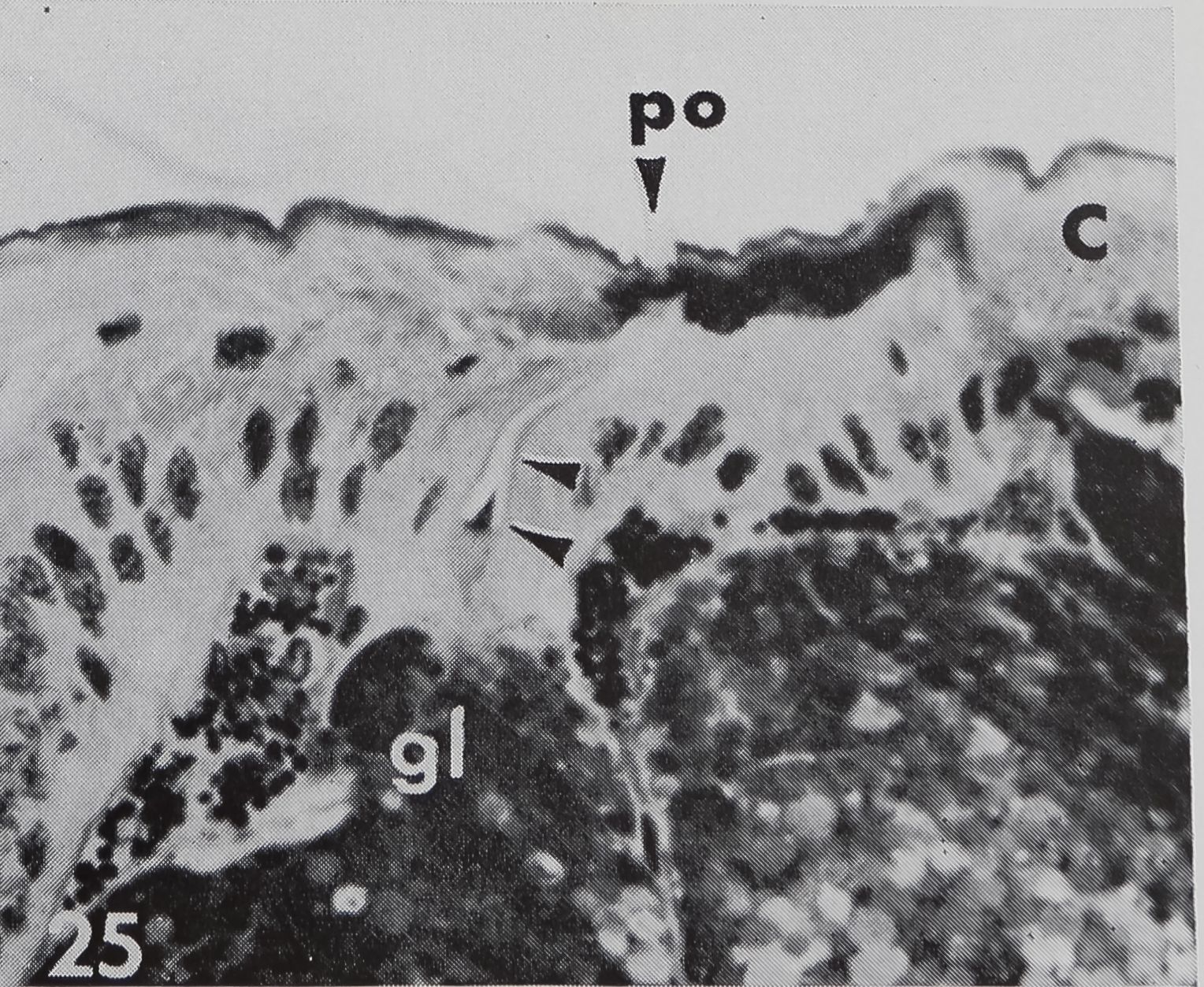
There are several organs on a spider’s leg which were previously described as being chemoreceptors by different authors : (1) lyriform organs (Mclndoo, Tl; Kas-ton, ’35); (2) single slit sensilla (Kaston, ’36; Keller, ’61), (3) tarsal organ (Blumen-thal, ’35) and (4) a pore plate in the mouth region (Dahl, 1885; Legendre, ’56; Keller, ’62). The first two possibilities are unlikely, as it is known now that lyriform organs (Liesenfeld, ’61; Walcott, ’69) as well as single slit sensilla (Barth, ’67) are mechanoreceptors. The tarsal organ, a small pit on the dorsal side of the tarsus (fig. 22), is multiply innervated and probably open the the outside; electron micrographs (own unpublished observations) show several dendrites enclosed in a “cutic-ular sheath” penetrating the thin cuticle which lines the pit. Each cuticular sheath contains several dendritic fibers, probably branches of one single dendrite. The structure of the tarsal organ provides strong evidence for some sort of chemoreception. Blumenthal (’35) states that no response to water and odor occurs, when this organ is covered with vaseline on all legs. Later Legendre (’56) contradicted Blumenthal’s results and described an organ located at ‘la jonction rostro-p alp aire” as the olfactory organ. A pore plate could be found on the inner side of the maxillae (figs. 23, 24) which corresponds probably to that organ, as well as the one described by Dahl in 1885. From the position of that organ close to the mouth opening, it seems likely to be a taste receptor, but in Araneus these pores represent glandular openings of a maxillar mucus gland (fig. 25). In another spider, Cupiennus, Keller (’62) reported a pore field on the maxilla which contains more than 100 taste receptors and only few glandular openings. In a similar location, on the basal segments of the chelic-erae, a taste receptor organ was found in scorpions (Alexander and Ewer, ’57).
As a working hypothesis one could speculate that the tarsal organ is olfactory in function, some pores in the mouth region or esophagus subserve taste reception, and the curved, blunt-tipped hairs are contact chemoreceptors. Most of the former authors assumed only one “chemoreceptor,” thus excluding the results of other workers. By means of elec trophy siological tests, one
could more clearly relate the possible chemoreceptor organs to their specific function.
A chemical sense has been demonstrated by behavioral experiments in many other orders of arachnids (Acarina, Scorpiones, Pseudoscorpiones, Opiliones and Solifu-gae) but the receptors have rarely been identified. In ticks and mites chemorecep-tive organs are located in setae of the first walking legs (Schulze, ’41; Lees, ’48; Camin, ’53). Recent ultrastructural investit gâtions on certain ticks (Coons and Ax-tell, personal communication) revealed bristles with several dendrites inside the lumen and a pore system in the cuticle wall. In scorpions a batch of bristles on the second cheliceral segment is considered to function as contact chemoreceptor (Abushama, ’64). Extensive behavioral experiments with harvest-men (Immel, ’54 B demonstrate their sense of smell and taste; the corresponding receptors are probably located on the palps and in the mouth region, but the specific organs are not known.
In spite of the limited knowledge it seems that in arachnids, as in other Arthropods, modified hairs are involved in chemoreception. Several different hair types have been reported for spiders (Dahl, ’20 B Gossel, ’35) but a chemoreceptive function was not indicated previously. The present work provides the morphological basis for detailed physiological experiments.
ACKNOWLEDGMENTS
The author is grateful to Dr. P. N. Witt and Dr. E. H. Slifer for valuable discussions and advice. Mr. G. Ross (Chem-strand) and Dr. R. C. Axtell (N. C. State University) kindly offered their help for scanning electron microscopy. This work was carried out in the Research Division of the North Carolina Department of Mental Health and supported in part by National Science Foundation grant GB-6246X1, to Dr. P. N. Witt. This research was also aided by contract N00014-70-A-0120-001 (NR 306-033), between the Office of Naval Research, Department of the Navy, and North Carolina State University.
LITERATURE CITED Abushama, F. T. 1964 On the behaviour and
sensory physiology of the scorpion Leiurus
320
RAINER F. FOELIX
quinquestriatus (H, & E.). Anim. Behav., 12: 140-153.
Adams, J. R., P. E. Holbert and A. J. Forgash 1965 Electron microscopy of the contact chemoreceptors of the stablefly, Stomoxys calci-trans (Diptera: Muscidae). Ann. Entomol. Soc. Am., 58: 909-917.
Alexander, A. J., and D. W. Ewer 1957 A chemoreceptor in the scorpion Opisthophtal-mus. S, Afr. J Sci., 53: 421-422.
Barth, F. G. 1967 Ein einzelnes Spaltsinnesor-gan auf dem Spinnentarsus; Seine Erregung in Abhângigkeit von den Parametern des Luft-schallreizes. Z. vergl. Physiol., 55: 407-449.
Blumenthal, H. 1935 Untersuchungen über das “Tarsalorgan” der Spinnen. Z. Morph. Ôkol. Tiere, 29: 667-719.
Bristowe, W. S. 1958 The world of spiders, Collins, London, pp. 34, 55, 138, 160.
Bristowe, W. S., and G. H. Locket 1926 The courtship of British lycosid spiders and its probable significance. Proc. Zool. Soc. London,I 1926: 317-347.
Camin, J. H. 1953 Observations on the life history and behavior of the snake mite Ophio-nyssus natricis (Gervais). Chicago Acad. Sci. Special Publ., 10: 1-75.
Corbière, G. 1969 Ultrastructure des sensilles trichoïdes de Tantenne chez les larves de Spe-ophyes lucidulus (Delar.) (Col. Bathysc.), C. R. Acad. Sc. Paris, 268: 387-388.
Dahl, F. 1885 Das Gehôr- und Geruchsorgan der Spinnen. Arch. Mikr. Anat., 24: 1-11.
– 1920 Die Sinneshaare der Spinnenti-
ere. Zool. Anz., 51: 215-219.
Dethier, V. G. 1955 The physiology and histology of the contact chemoreceptor of the blow fly. Quart. Review Biol., 30: 348-371.
– 1963 The physiology of insect senses.
Methuen, London, pp. 112-155.
Eberhard, W. G. 1969 The spider Uloborus diver sus (Marx) (Uloboridae) and its web. Doct. thesis. Harvard Univ., Cambridge, Mass.
Ernst, K. D. 1969 Die Feinstruktur von Riech-sensillen auf der Antenne des Aaskâfers Ne-crophorus (Coleoptera). Z. Zellforsch., 94: 72-102.
Foelix, R. F. 1970 Structure and function of tarsal sensilla in the spider Araneus diadema-tus. J. Exp. Zool., in press.
Gossel, P. 1935 Beitrâge zur Kenntnis der Hautsinnesorgane und Hautdrüsen der Chelic-eraten. Z. Morph. Ôkol. Tiere, 30: 177-205.
Grabowski, C. T., and V. G. Dethier 1954 The structure of the tarsal chemoreceptors of the blow fly, Phormia regina Meigen. J. Morph., 94: 1-19.
Heil, K. H. 1936 Beitrâge zur Physiologie und Psychologie der Springspinnen. Z. vergl. Physiol., 23: 1-25.
Homberg, P. 1707 Observations sur les araignées. Mem. Acad. France; cit. Blumenthal, “35.
Hodgson, E. S. 1958 Chemoreception in arthropods. Ann. Rev. Entom., 3: 19-36.
— 1965 The chemical senses and changing viewpoints in sensory physiology. Viewpoints in Biol., 4: 83-124.
– 1968 Taste receptors in arthropods.
Symp. Zool. Soc. Lond. No. 23: 269—277.
Hodgson, E. D., and K. D. Roeder 1956 Elec-trophysiological studies of arthropod chemoreception. I. General properties of the labellar chemoreceptors of diptera. J. Cell, and Comp. Physiol., 48: 51-75.
Hodgson, E. S., J. Y. Lettvin and K. D. Roeder 1955 Physiology of a, primary chemoreceptor unit. Science, 122: 417—418.
Immel, V. 1964 Zur Biologie und Physiologie von Nemastoma quadripunctatum ( Opiliones, Dyspnoi). Zool. Jahrb. Abt. Syst., 83: 129-184.
Kaston, B. J. 1935 r The slit sense organ of spiders. J. Morph., 58: 189—209.
– 1936 The sense inlvoved in the courtship of some vagabond spiders. Entom. Am., 16: 97-167.
Keller, L. R. 1961 Untersuchungen fiber den Geruchssinn der Spinnenart Cupiennus salei Keyserling. Z. vergl. Physiol., 44: 576—612.
– 1962 Über den Geschmackssinn der
Spinne Cupiennus salei Keyserling. Biol. Zbl., 81: 315-320.
Larsen, J. R. 1962 The fine structure of the labellar chemosensory hairs onthe blowfly Phormia regina Meigen. J. Insect Physiol., 8: 683-691.
Lees, A. D. 1948 The sensory physiology of the sheep tick, Ixodes ricinus L. J. Exp. Biol., 25: 145-207.
Legendre, R. 1956 Localisation d’un organe olfactif non encore décrit chez les aranéides. C. R. Acad. Sci. (Paris), 243: 1237-1240.
Liesenfeld, F. 1961 Über Leistung and Sitz des Erschütterungssinnes von Netzspinnen. Biol. Zbl., 80: 465-475.
McCook, H. C. 1890 American spiders and their spinning work. Vol. II. Publ. by author and Acad. Nat. Sci. (PhiladelphiaBp. 299.
Mclndoo, N. E. 1911 The lyriform organs and tactile hairs of araheads. Proc. Acad. Nat. Sci. (Philadelphia), 63: 375-418.
Mellon, D., and D. R. Evans 1961 Electrophysi-ological evidence that water stimulates a fourth sensory cell in the blowfly taste receptor. Am. Zool., 1: 372.
Millonig, F. 1961 Advantages of a phosphate buffer for OsC>4 solutions in fixation. J. Appl. Phys., 32: 1637.
Millot, J. 1936 Le sens du goût chez les araignées. Bull. Soc. Zool. France, 61: 27—38.
– 1946 Sens chimique et sens visuel chez
les araignées. Ann. Biol., 22: 1-21.
Pease, D. C. 1964 Histological techniques for electron microscopy. 2nd ed. Acad. Press, New York, p. 229.
Peters, W. 1965 Die Sinnesorgane an den Label-len von Calliphora erythrocephala MG (Diptera). Z. Morph. Ôkol. Tiere, 55: 259—320.
Pritchett, A. H. 1904 Observations on hearing and smell in spiders. Am. Naturalist, 38: 859— 867.
Richardson, K, C., L. J. Jarret and E. H. Finke 1960 Embedding in Epoxy resins for ultrathin sectioning in electron microscopy. Stain Tech-nol., 35: 313-323.
CHEMOSENSITIVE HAIRS IN SPIDERS
321
Rovner, J. S. 1968 An analysis of display in the lycosid spider Lycosa rabida Walckaer. Anim. Behav., 16: 358-369.
Sabatini, D. D., K. Bensch and R. J. Barrett 1963 Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell Biol., 17: 19-58.
Schneider, D. 1969 Insect olfaction: deciphering systems for chemical messages. Science, 163: 1031-1037.
Schulze, P. 1941 Das Geruchsorgan der Zeck-en. Untersuchungen fiber die Abwandlungen eines Sinnesorgans und seine stammesgeschicht-liche Bedeutung. Z. Morph. Ôkol. Tiere, 37: 491-564.
Slifer, E. H. 1960 A rapid method for identifying permeable areas of the body wall of insects. Entomol. News, 71: 179-182.
– 1961 The fine structure of insect sense
organs. Intern. Rev. Cytol., 11: 125—159.
1968 The thin-walled olfactory sense organs on insect antennae. In: Insects and Physiology. Beament and Treherne, eds. Elsevier, New York, pp. 233—245.
Slifer, E. H., J. J. Prestage and H. W. Beams 1957 The fine structure of the long basiconic sensory pegs of the grasshopper (Orthoptera: Acrididae) with special reference to those on the antenna. J. Morph., 101: 359—397.
— 1959 The chemoreceptors and other
sense organs on the antennal flagellum of the grasshopper (Orthoptera: Acrididae). J. Morph., 105: 145-191.
Stfirckow, B. 1967 Occurrence of a viscous substance at the tip of the labellar hair of the blowfly. In: Olfaction and taste. T. Hayashi, ed. Pergamon Press, New York, 2: 707—720.
Walcott, C. 1969 A spider’s vibration receptor: its anatomy and physiology. Am. Zool., 9: 133-145.
Wilczek, M. 1967 The distribution and neuroanatomy of the labellar sense organs of the blow fly Phormia regina (Meigen). J. Morph., 122: 175-201.
Wolbarsht, M. D., and V. G. Dethier 1958 Electrical activity in the chemorecptors of the blowfly. I. Responses to chemical and mechanical stimulation. J. Gen. Physiol., 42; 393—412.
PLATE 1
EXPLANATION OF FIGURES
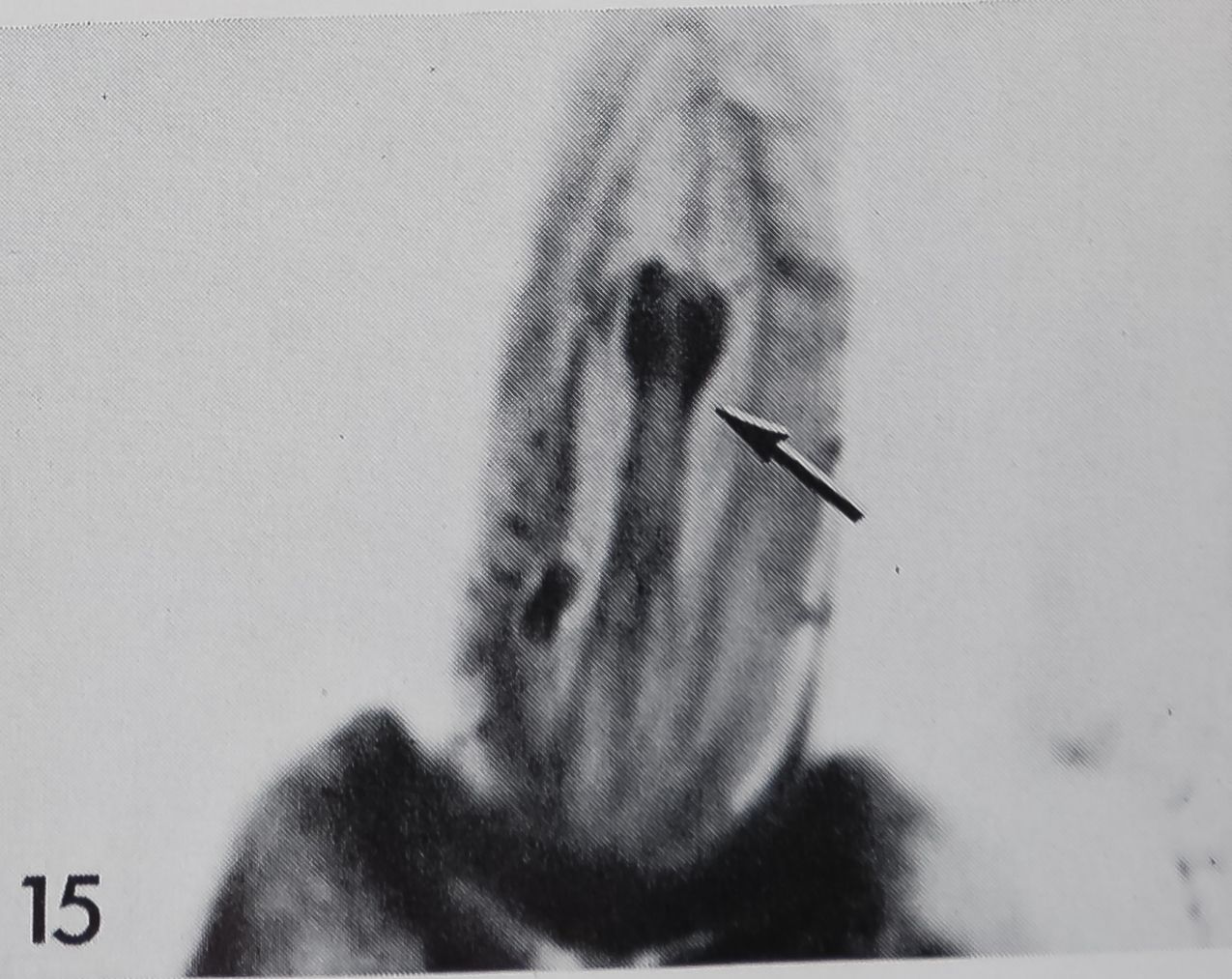
3 Two “chemosensitive” hairs on the ventral side of the tarsus of Araneus diadematus (arrows). Note the steep angle with respect to the leg axis, the slight curvature and blunt tips as compared to surrounding tactile hairs* 390.
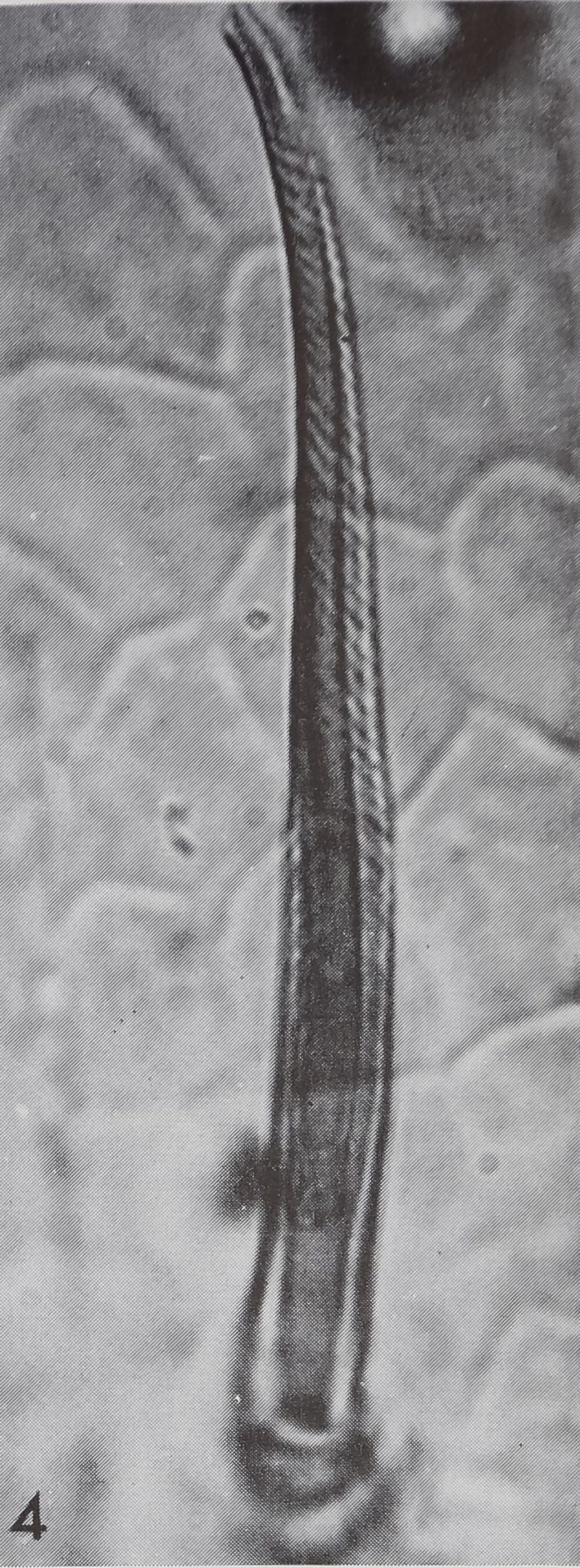
4 “Chemosensitive” hair of an exuvia of Araneus diadematus. Surface striation, double lumen and relatively thin walls are typical. The polygonal platelets in the background picture the surface of the leg cuticle. Hl.300.
5 Three different kinds of hairs are found on the edge of the maxillae of Araneus diadematus. Thin, feather-like hairs (f), solid hairs with a crown of little spines (s) and curved, “chemosensitive” hairs (c). Only the last type is innervated. X 430.
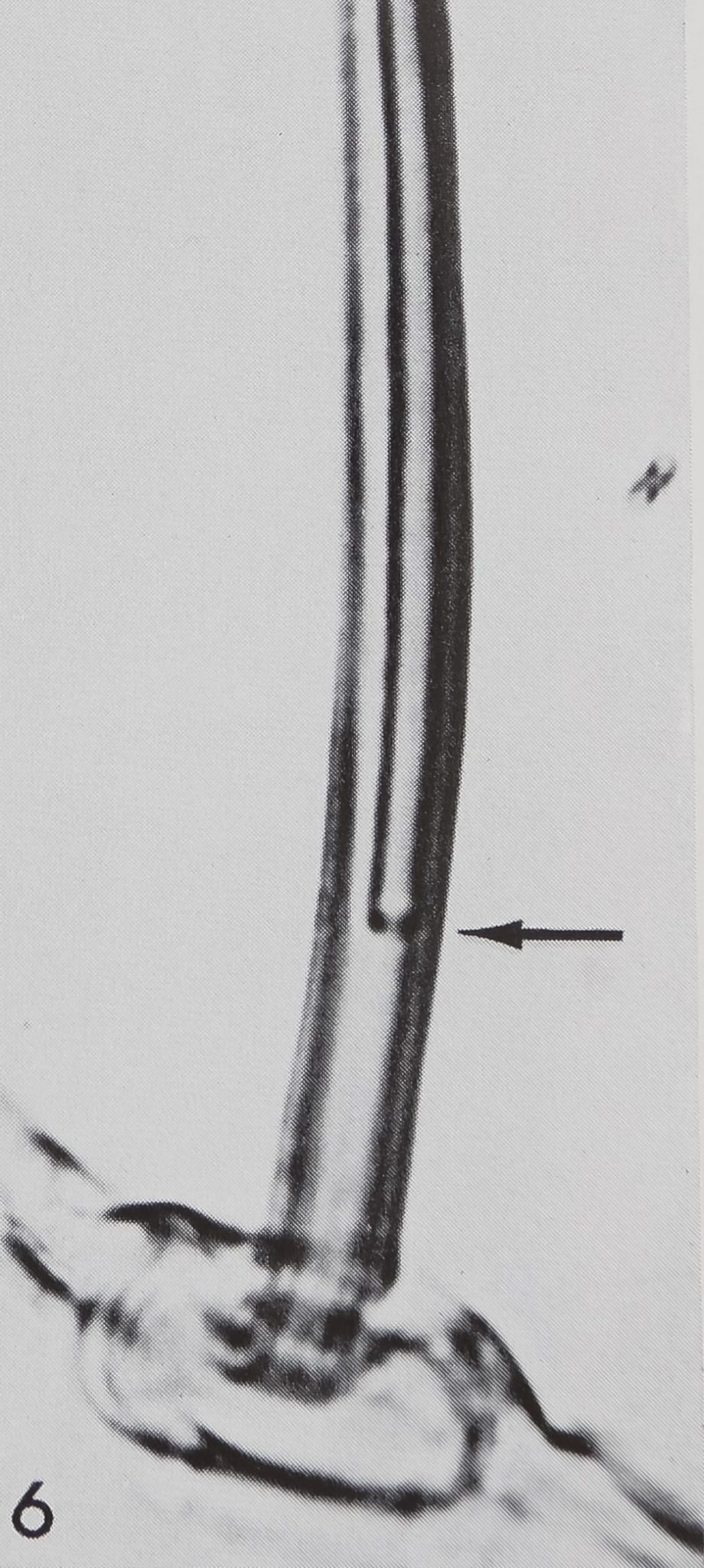
6 Higher magnification of “chemosensitive” hairs on the maxilla shows typical double lumen but no surface striation. Note the constriction at the beginning of the cuticular tube inside the hair lumen (arrow).
IBM80–
CHEMOSENSITIVE HAIRS IN SPIDERS Rainer F. Foelix




PLATE 2
EXPLANATION OF FIGURES
7 Scanning electron micrograph (SEM) of the tip of a “chemosensitive” hair in Argiope aurantia. Note the oval opening to the outside (arrow).
-K 16,500.
8 SEM of the tip of a “chemosensitive” hair in Araneus diadematus. The blunt tip shows a depression which represents the orifice of the cuticular tube. JÈ 12,500.
9 Light micrograph of the tip of a “chemosensitive” hair. This transparent exuvia indicates a direct communication of the cuticular tube to the outside (arrow). JÊ 2,700.
10 A broken shaft of a “chemosensitive” hair (exuvia) demonstrates the double lumen. Only the circular lumen (tube) contains nerve fibers. The cristae are restricted to one side of the hair; this cannot be recognized in the light microscope (compare fig. 9). SEM X 9,800.
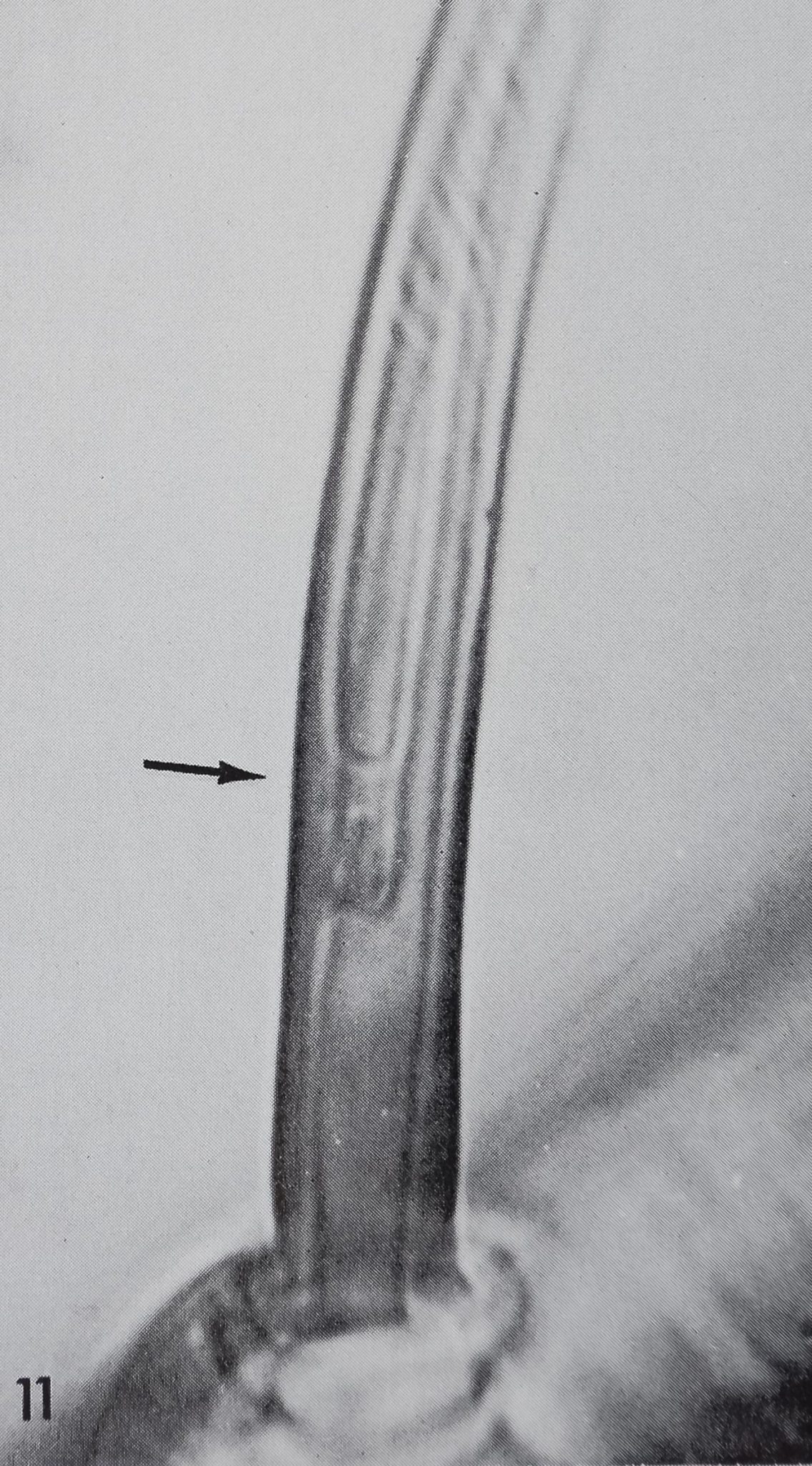
11 Base of a “chemosensitive” hairHexuvia) in Araneus diadematus. Note the attachment of the cuticular tube to the hair wall about 10 /jl above the hair base (arrow) and the smooth surface of that lower part of the hair. 2,300.
CHEMOSENSITIVE HAIRS IN SPIDERS Rainer F. Foelix
PLATE 2
325





PLATE 3
EXPLANATION OF FIGURES
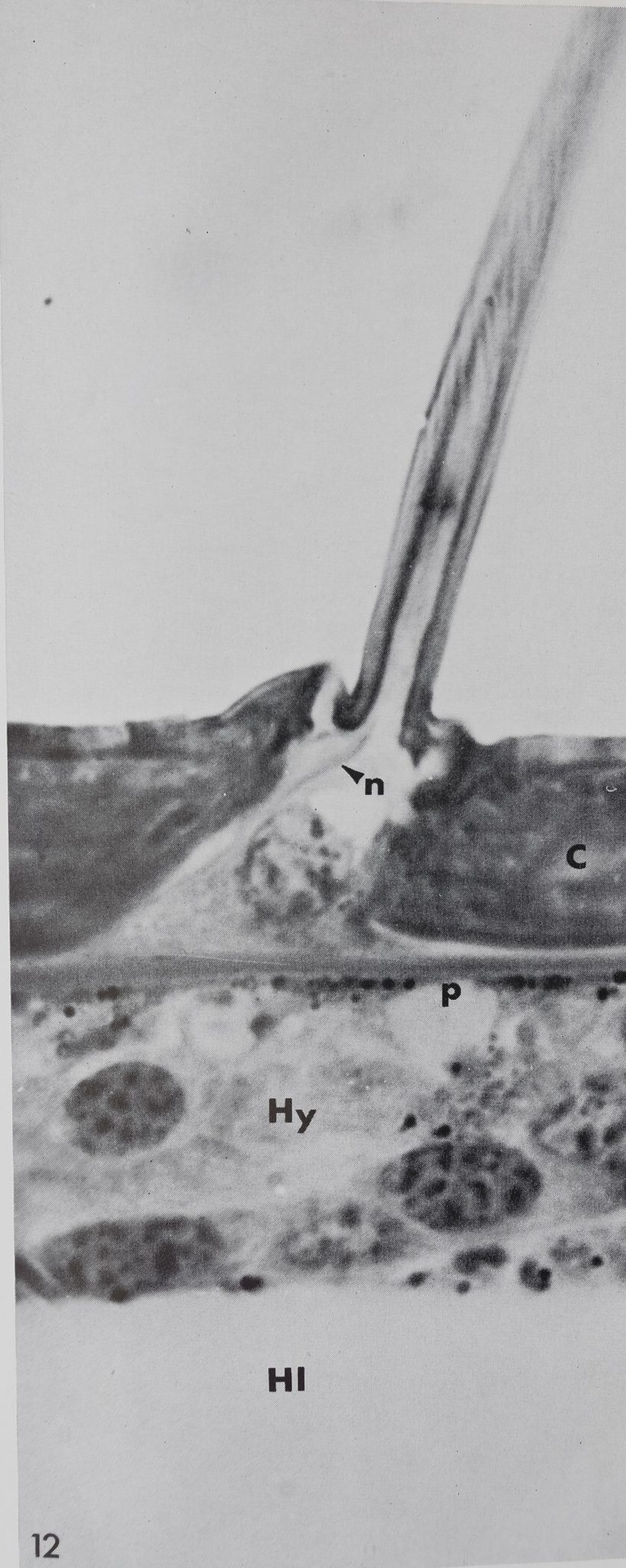
12 Longitudinal section of a “chemosensitive” hair in an adult Araneus diadematus. Nerve SbersBn) enter the hair lumén proximally, C, cuticle; p, pigment gfmnul##.;: By* ■ hypodermis; HI, hemolymph’,
Û 2,200.
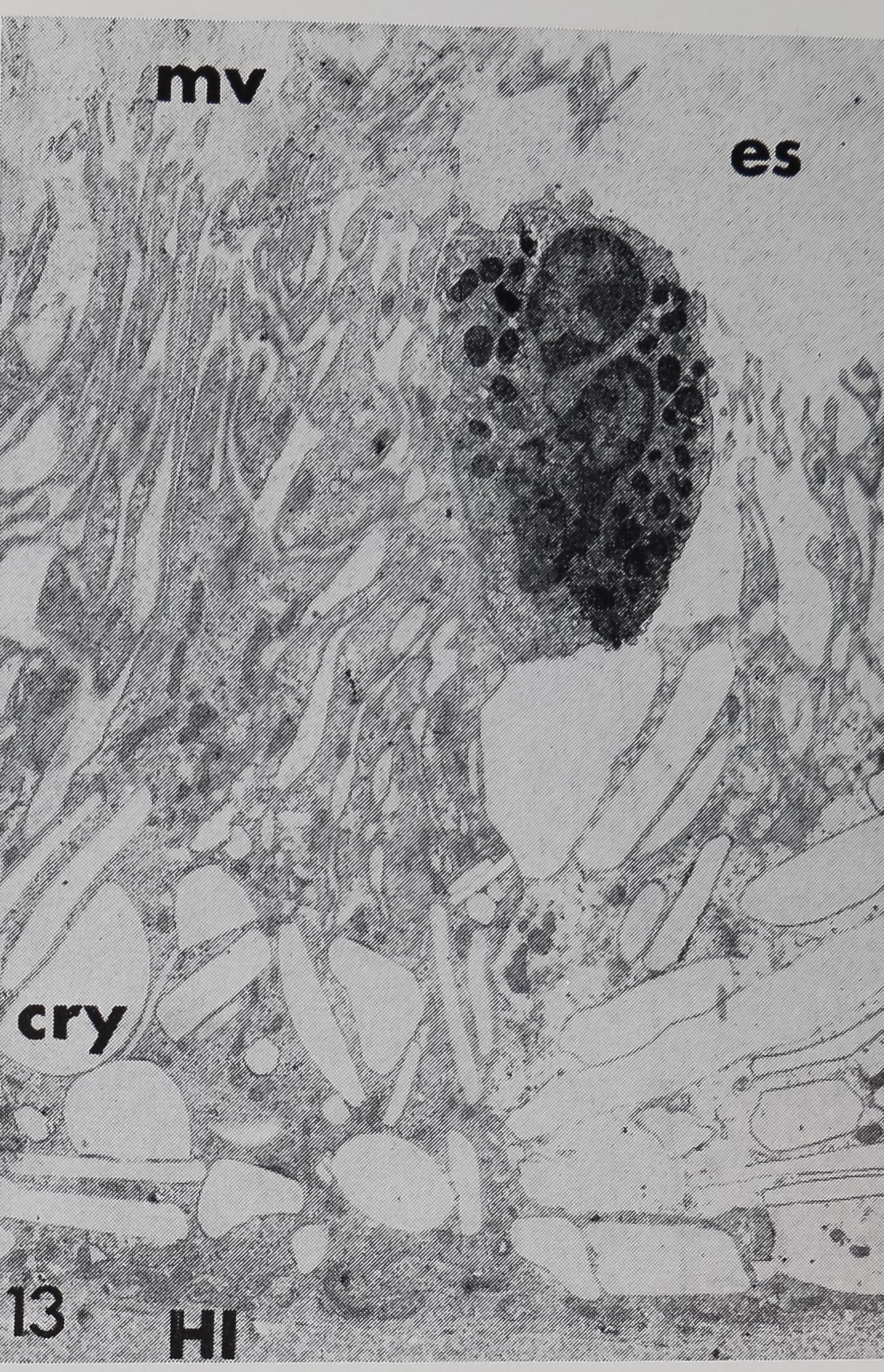
13 Electron micrograph of the hypodermis cell Underlying the hair base. Numerous microvilli (mv) extend into the extracellular space (es); note the hemolymph cell with a constricted nucleus and many granules, which is attached to the apical cell surface. The basal part of the cell is filled by crystalline deposits (cry).® 3,700.
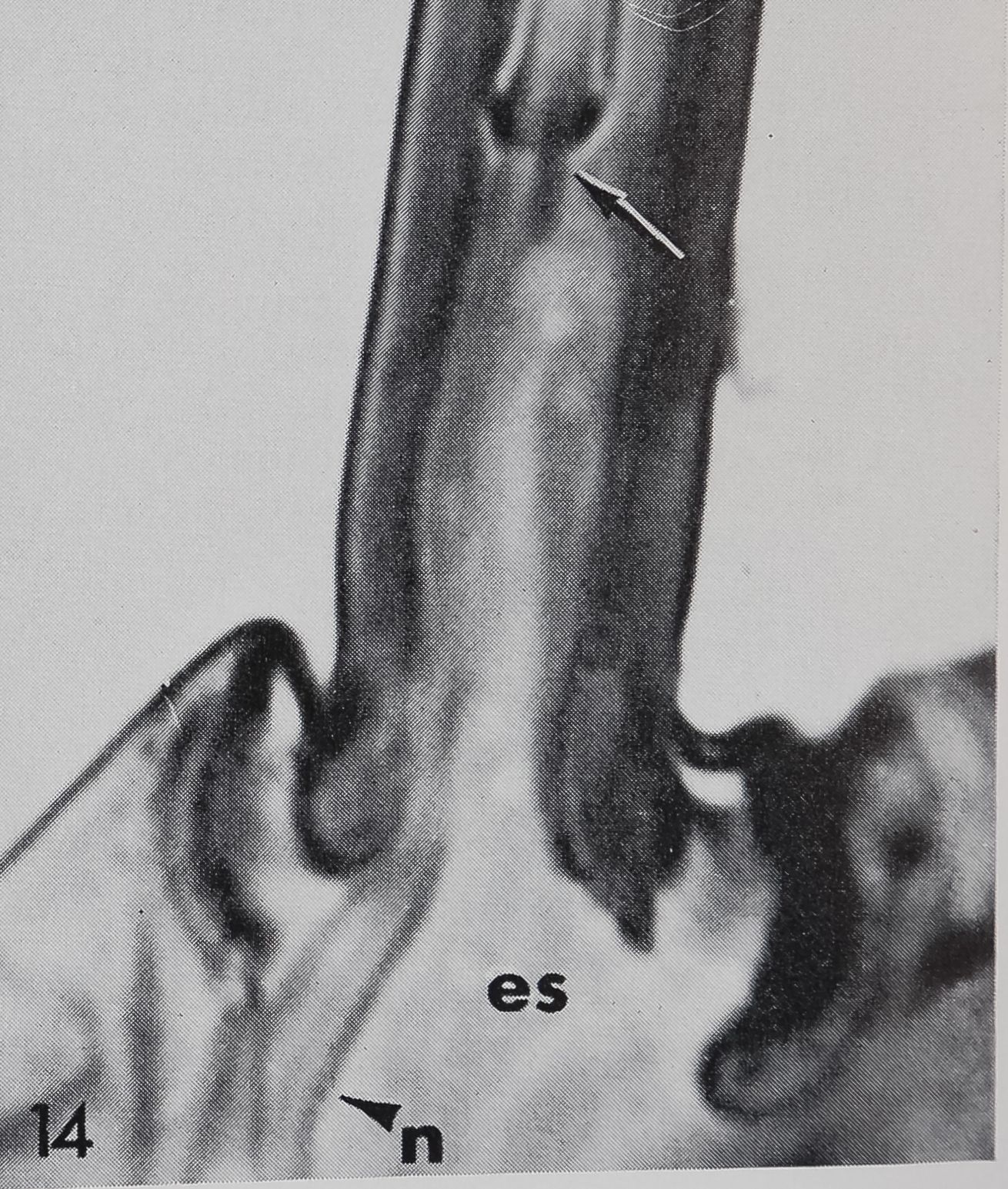
14 The nerve fibers (n) follow the proximal cuticle wall and enter the cuticular tubepc arrow -Kr 2,800.
15 Oblique section of a “chemoseniitS#- hair showing two dendrites penetrating the cuticular tube (arrow500.
326
CHEMO SEN SUIVE Rainer F. Foelix
PLATE 3
327




PLATE 4
EXPLANATION OF FIGURES
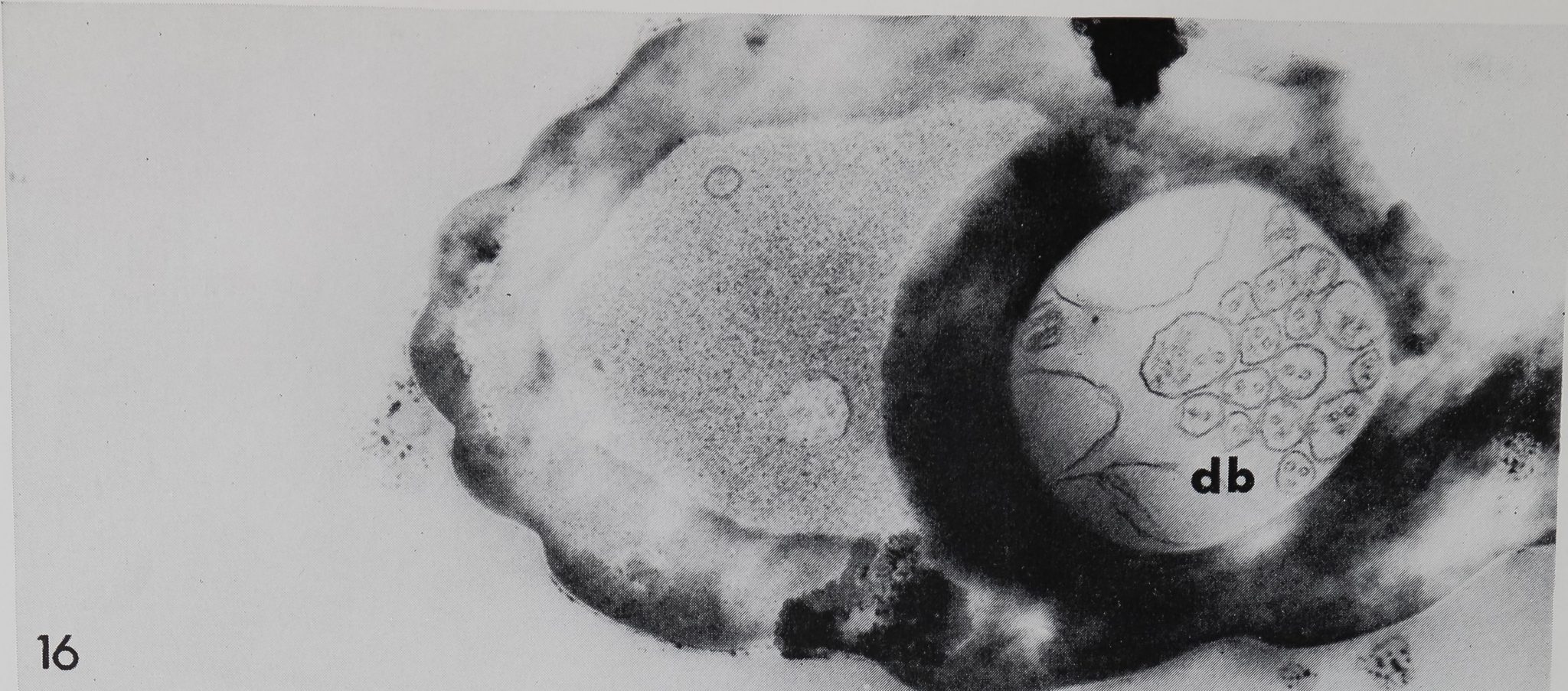
Electron micrograph of a cross section through a “chemosensitive” hair of an adult Araneus diadpmatus. The cuticular tube contains 17 dendritic branches Bdb), each with one or several microtubules, which are bathed in a homogeneous fluid. The crescent-shaped lumen is also fluid-filled but appears granular and may contain some; vacuoles, m 40,000.
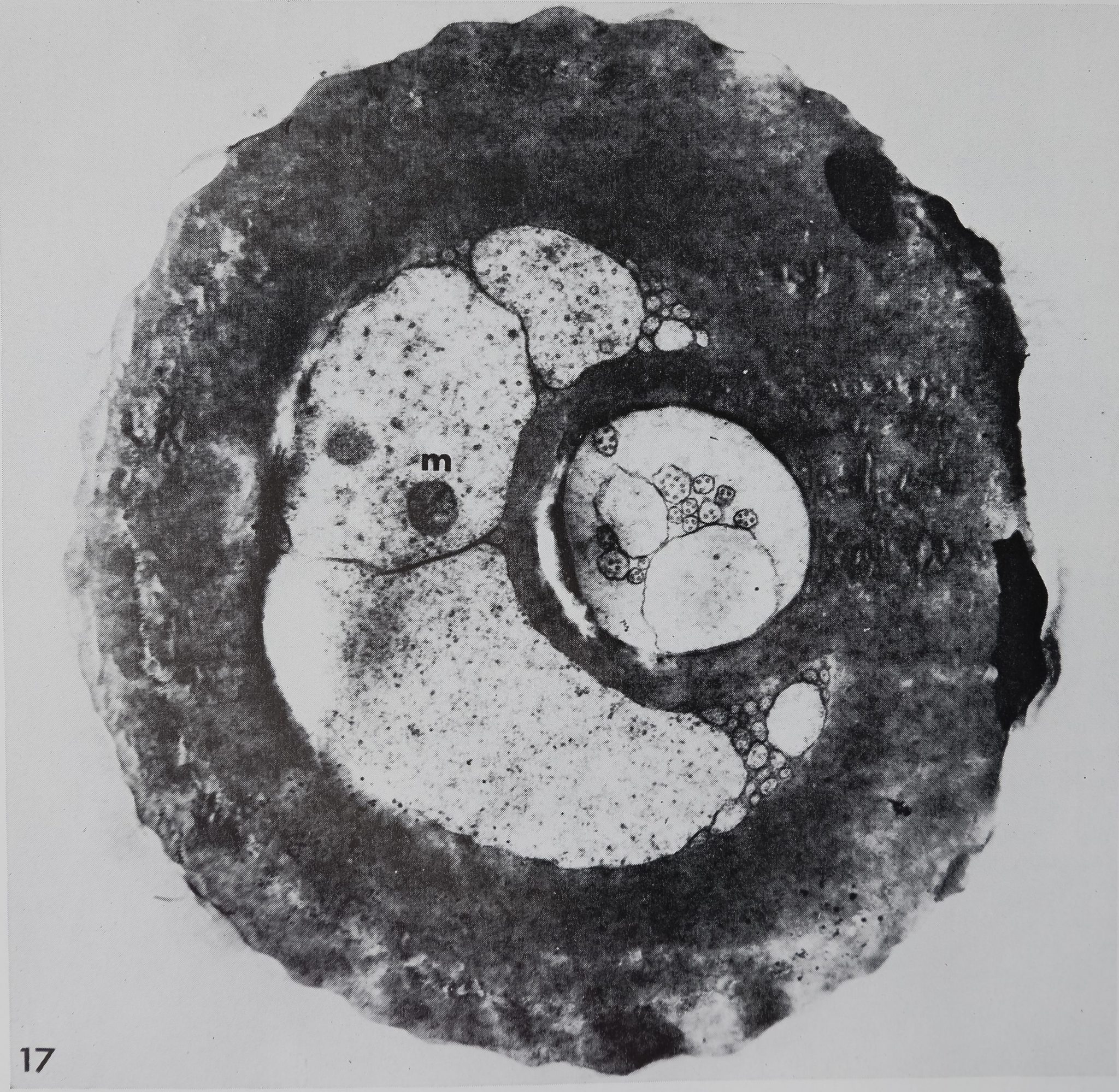
Cross section of a “chemosensitive” hair of Araneus diadematus shortly after molting. The crescent-shaped lumen is filled by processes of the trichogene cell while the dendritic branches occupy the cuticular tube. Note the absence of any pore system in the cuticle of the hair wall, m, mitochondrium. x 21,000.
CHEMOSENSITIVE HAIRS IN SPIDERS Rainer F. Foelix
PLATE 4

329

PLATE 5
EXPLANATION OF FIGURES
Tip of the leg of a young wolf spider (Lycosa rabida ). The “chemo-sensitive” hair is easily recognizable by its shape, surface structure and the \double lumen (arrow).^Bp30.
Ventral surface of a tarsus in a Frjkisiata sp. ( Filistatidae )g| as seen with the scanning electron microscope. At least two classes of brushlike bristles can be distinguished, which are probably tactile in function. The “chemo sensitive” hairs are arranged in a row between them and can be recognized by their slender shaft and the bent tip (arrow). B 230.
Close-up of figure 19 showing the blunt tip of a “chemosensitive” hair. In contrast to the surrounding common hairs it bears almost no surface structures^H750.
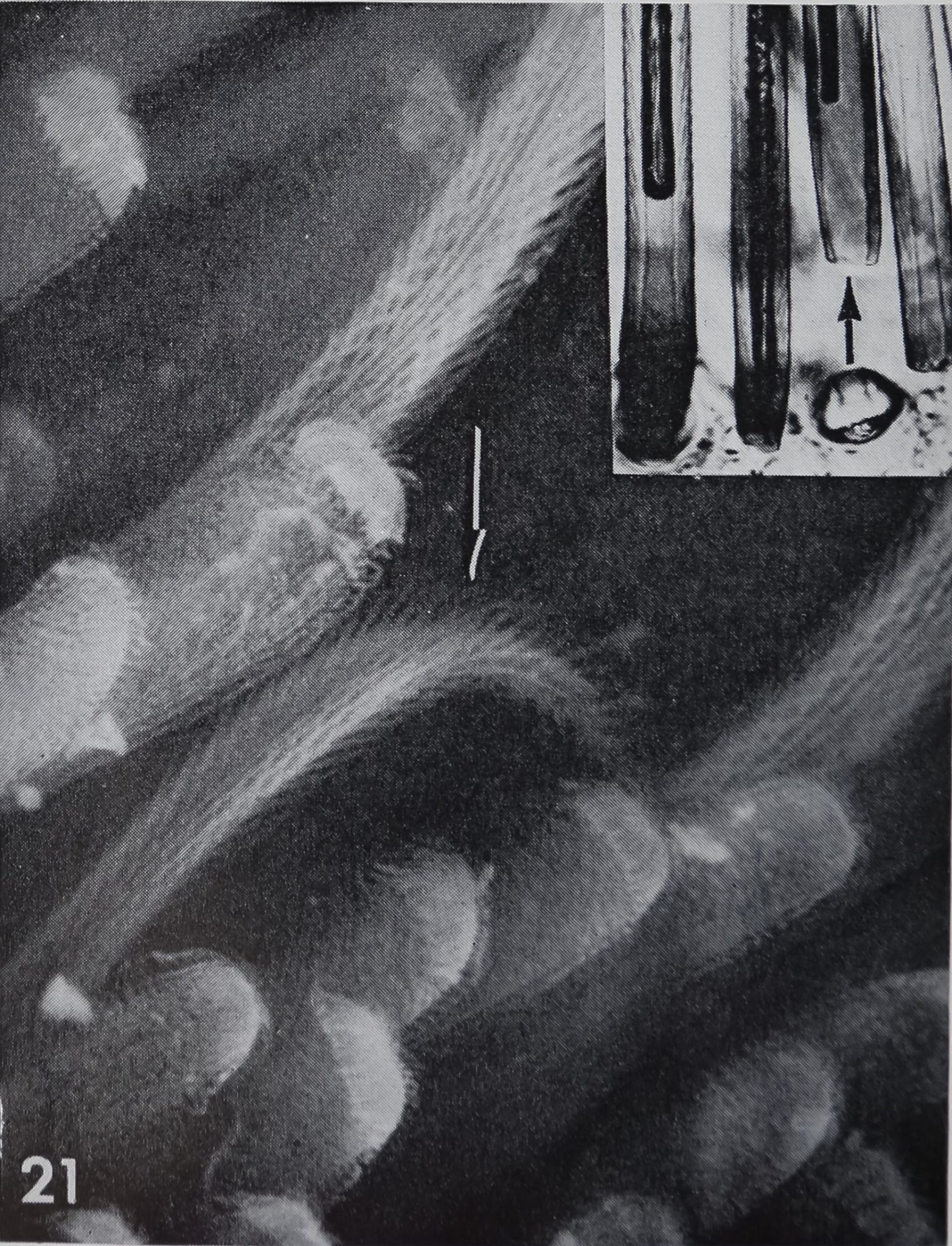
The scopula on the tip of the tarsus in Cupiennus salei consists of tightly packed hairs and only few “chemosensitive” hairs can be seen (arrow). SEM, BBl,000. Insert: Whole mounts of exuviae demonstrate the double lumen and the relatively thin wall of “chemosensitive” hairs (arrow).B 360.
CHEMOSENSITIVE HAIRS IN SPIDERS Rainer F. Foelix
PLATE 5
331




PLATE 6
EXPLANATION OF FIGURES
22 The tarsal organ in Arançus diadematus is a pit of about 30 n diameter on the dorsal surface of the tarsus. This SEM pictures the distinct cuticular ring covering the pit. Note the scaly appearance of the leg cuticle and the surface structure-of tactile bristles. Mr 1,600. Insert: Electron micrograph of a longitudinal section of the tarsal organ in Araneus diadematus. Several teeth emerge from the bottom of the groove, which is lined by a thin cuticle. Six or seven nerve fibers, enclosed by a cuticular sheath, are seen penétrating this cone. As they probably communicate with the outside (arrows), the tarsal organ seems to be a chemoreceptor^Hl,750.
23 Maxilla (Mx) and inserting pedipalp (p) in Araneus diadematus, seen from the inner side. A small pore plate, indicated by the arrow, might be a chemoreceptor. The edge of the maxilla bears about a dozen “chemosensitive” hairs (compare figs. 5, 6).ft 45.
24 A scanning electron micrograph of the pore plate shows 13 openings of about 1 fi diameter and many cuticular foldings between the pores.
K 900.
25 A histological section of the pore plate in Araneus diadematus demonstrates that the pores are openings of a maxillar mucus gland (gl) and are not supplied by sensory cells. An excretory duct (two arrows) connects the pore (po) with the gland. C, cuticle. ^ 410.
CHEMOSENSITIVE HAIRS IN SPIDERS Rainer F. Foelix
PLATE 6
333