Images Collection
View this article in Search Friendly Plain Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
an Article from
SCIENTIFIC
AMERICAN
234,
NO. 3
Social Spiders
Most adult spiders lead solitary lives. A few species, however, are
gregarious and others even build large communal webs. Both degrees
of spider sociality can be observed among species native to Mexico
by J. Wesley Burgess
Among insects—notably bees, ants and
M termites—social behavior is com-
-*• *** mon. Among spiders it is rare. All
spiders are predatory carnivores; among
many of them even the male of the species
cannot approach the female without risk of
being attacked and killed. It is therefore
paradoxical that there are any social spiders
at all. How, then, can such spiders exist?
The number of social spiders || small;
only in 12 genera distributed among nine
families of spiders is any kind of sociality
known. The 12 genera are, however, widely
distributed, with representatives in both the
Old World and the New. Two of the New
World species are found in Mexico. I re-
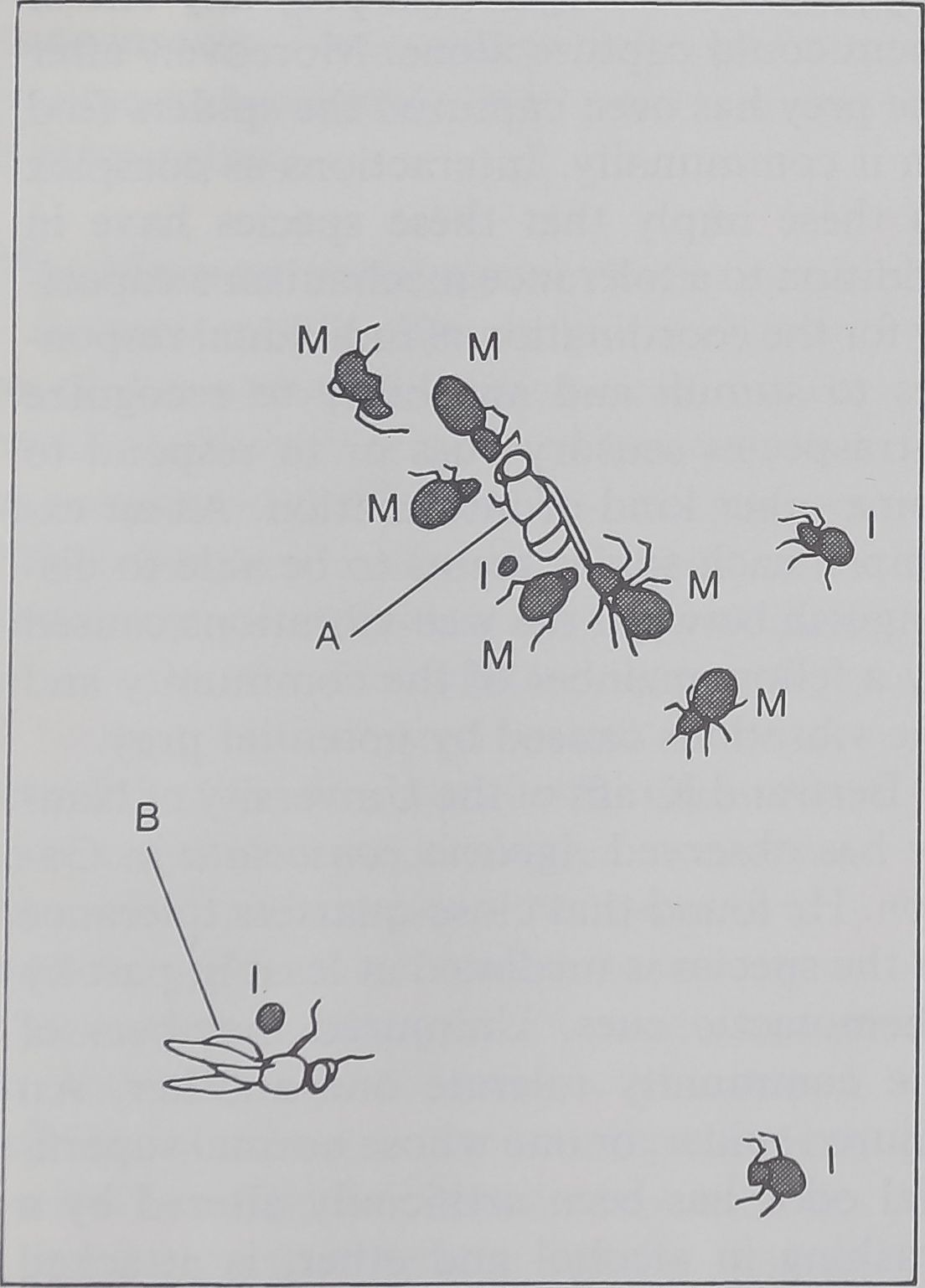
COOPERATIVE CAPTURE of a fly (A) by
several spiders is seen in the photograph on
the opposite page. The diagram above identi-
fies prey and predators. Spiders labeled M
are mature; those labeled I are immature.
Only two of the many flies on the web (A, and
B at bottom left) are new catches. The spiders
are of the social species Mallos gregalis. The
cluster of mature spiders is feeding or prepar-
ing to feed. One immature spider has been
drawn to the scene; another is approaching.
The photograph was made in the author’s
laboratory; spiders were collected in Mexico.
cently visited areas near Guadalajara where
both species are present, observed the social
spiders in their natural habitat and brought
home to North Carolina a number of speci-
mens for rearing and further observation in
the laboratory.
The two Mexican species lead distinctive-
ly different lives. Mallos (formerly Coeno-
thele) gregalis is a small spider, with a body
that rarely exceeds five millimeters in
length. It builds a large colonial web, sur-
rounding the branches of a tree with a con-
tinuous sheet of silk. Its aggregations may
be socially the most complex spider colonies
in North America. Oecobius civitas is an
even smaller spider; few have bodies more
than two and a half millimeters long. It lives
gregariously, spinning its silk shelter and
alarm-system web in a dark and narrow
rhicrohabitat: the underside of a rock.
Spider societies are different from the so-
cieties found among the higher social in-
sects both in kind and ra degree. One reason
is that a spider’s web extends its range of
sensory perception in a way that has no
analogy among insects. Another is that the
structure of a spider’s mouthparts is such
that ml can feed only on other animal life.
Any animal of appropriate size that a spider
encounters, including a spider of another
species or even the same species, is potential
prey. It will nonetheless be useful in de-
scribing the sociality of the social spiders to
sketch the probable evolution of different
degrees of sociality among insects.
As Edward O. Wilson of Harvard Uni-
versity has pointed out, the eusocial insects,
or higher social insects, have three traits in
common: cooperative care of the young, a
division of labor whereby more or less ster-
ile individuals attend to the needs of fertile
individuals, and a life cycle long enough for
the offspring at some point to share the ac-
tivities of the parental generation. The evo-
lutionary routes that may have led from
nonsocial to eusocial behavior appear to be
traceable in terms of the less than eusocial
behavior found among various insect rela-
tives of the eusocial species. Charles D.
Michener of the University of Kansas has
outlined two such possible routes.
The first route Michener calls parasocial;
on it there are three levels of increasingly
complex behavior on the way to eusociality.
The lowest level, communal behavior, is
characterized by an aggregation of female
insects, all belonging to the same genera-
tion; once the females have aggregated they
build a communal nest for their young. The
next level, quasi-social behavior, is charac-
terized by cooperative care of the young.
The third level, semisocial behavior, is char-
acterized by the appearance of different
castes that serve different roles. Thereafter
eusociality is achieved when the life cycle is
extended so that parents and mature off-
spring coexist in the same colony.
Michener’s second evolutionary route he
calls subsocial. On this route only one level
of behavior precedes eusociality; it is char-
acterized by solitary rather than communal
nest building. The solitary female remains
at the nest, however, and cares for her
young. Eusociality is achieved in one step
when the nest builder lives long enough to
have the assistance of its first daughter gen-
eration in caring for subsequent, caste-dif-
ferentiated daughter generations.
Looked at in these terms no social spider is
* eusocial. We must define the common
base of spider sociality in much more re-
stricted terms: the existence of various de-
grees of communality and of characteristic
interactions among the members of com-
munal aggregations.
Here it should be noted that with few
exceptions even spiders that are solitary in
habit go through a semicommunal stage
early in their life cycle. Unlike insects,
spiders do not have a larval stage. Each
emerges from the egg as a functioning mini-
ature adult, although it retains a yolk sac
that supplies it with nutrients for several
days. It grows in size and develops its sexual
characteristics through a series of succes-
sive molts, the earliest of which takes place
within the shelter of the parental egg sac. It
leaves the egg sac fully prepared to spin silk
and disable prey.
One might therefore expect that the spi-
derlings of the solitary species would scatter
as soon as they leave the egg sac. Instead for
the duration of a period known as the toler-
ant phase the spiderlings aggregate, and
many of them join in the labor of building a
small sheet web. They may even attack any
small prey animal that blunders into the
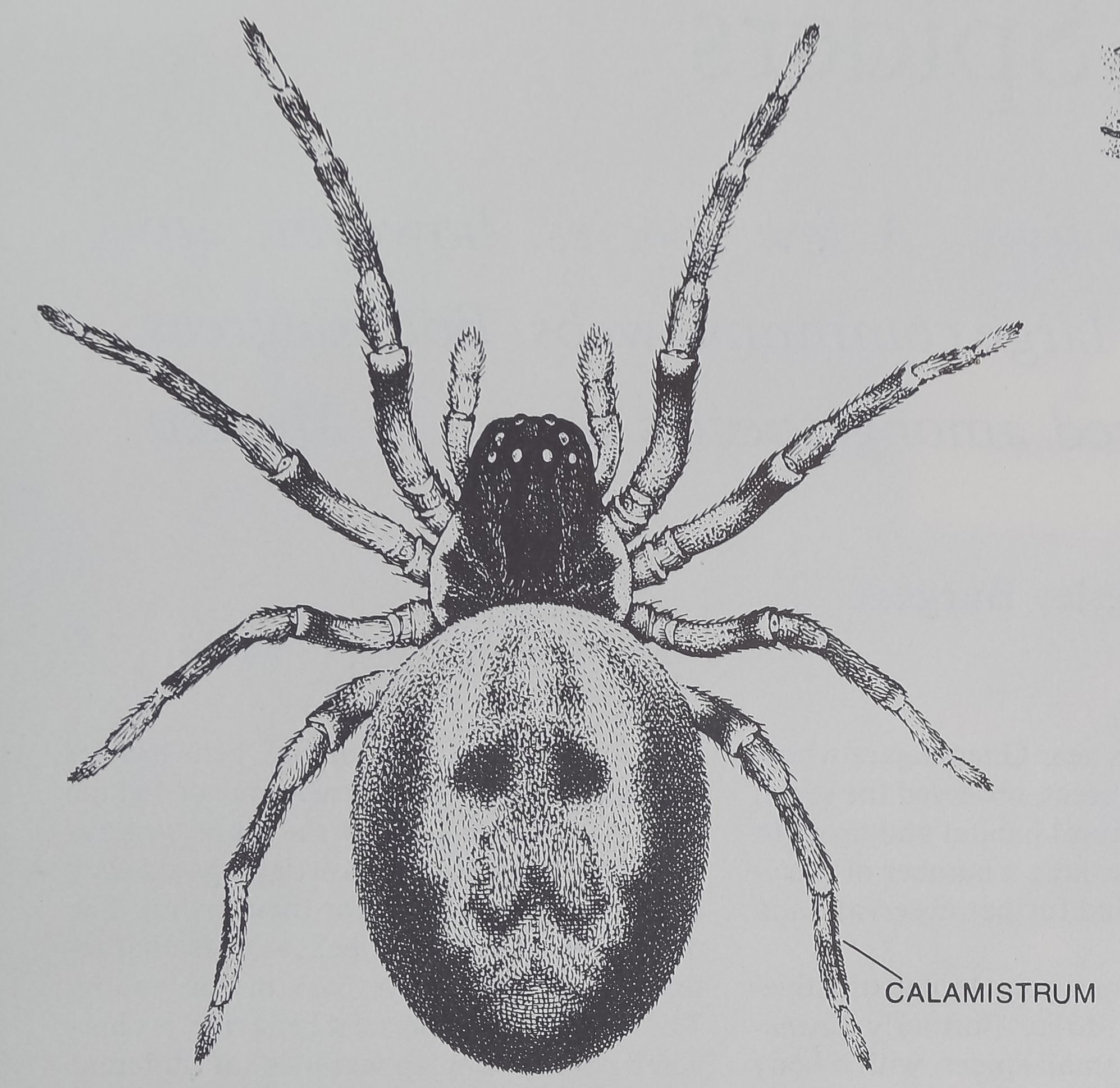
COMMUNAL SPIDER Mallos gregalis has an average body length
of five millimeters. Its complex flytrap web incorporates many sticky
bands of silk that entangle intruders. “The sticky Silk is drawn from
hundreds of microscopic pores in an abdominal plate (right), the
cribellum. The spider combs out the silk with its calamistrum, an ar-
ray of bristles that grows on the metatarsal of each of its hind legs.
web and wrap the intruder in silk. At this
early stage, however, they never feed on the
prey. After several days of the tolerant
phase have passed the spiderlings disperse,
build individual webs and feed on the prey
they capture. All the spiderlings appear to
adopt the solitary behavioral pattern simul-
taneously.
It is also noteworthy that in certain soli-
tary-spider species (including representa-
tives of the families Eresidae, Theridiidae
and Agelenidae) the adult female does not
abandon the egg sac after constructing it
but remains with it, or carries it with her,
until the spiderlings emerge. The female
may then allow them to share her captured
prey or may nourish them with regurgitated
food or special secretions. Such parental
care of the offspring bears a certain resem-
blance to the lower level of Michener’s sub-
social route to higher sociality! Thus even
among the spider species that are recog-
nized as being solitary, transient episodes
of sociality may be observed.
When spiders live in groups, a number of
additional interaction patterns are evident.
Spider groups form in a variety of way s. For
example, adult spiders of some species in
the families Uloboridae and Araneidae will
aggregate without regard to whether they
are the offspring of the same parents or dif-
ferent ones. Each individual in these aggre-
gations spins its own web. Among some
species the individual may also contribute
silk to a communal web area. Some of these
groups may be made up of as many as 1,000
adults. In general each individual lives inde-
pendently. All, however, share the benefits
of a large aggregate web surface and of mo-
nopolizing a habitat that might otherwise
have been shared by competitive species.
The viability of simple aggregations such
as these demonstrates the existence of a tol-
erance mechanism in the individual adult
spiders. At the very least the mechanism
must be strong enough to keep the spiders
from eating one another when prey are
scarce. Evidently the mechanism is also spe-
cies-specific; it is not limited to simply en-
suring that the spiders are tolerant of all the
other spiders in the aggregation. They are
also tolerant of any spider of their own spe-
cies. This has been demonstrated as follows.
Individuals of the species Metepeira spi-
ritpès, a member of the family Araneidae,
have been taken from populations living
hundreds of miles away and introduced into
local aggregations of M. spinipes. The pres-
ence of strangers did not disrupt the toler-
ance mechanism within the local aggrega-
tion, nor was any difference noted in the
behavior of the two groups.
The most dramatic examples of spider
sociality involve interactions substan-
tially more complex than those I have been
describing so far. These interactions are
known only for four (possibly five) spider
species. Two of the species are African:
Agelena consociata and Stegodyphus sarsi-
norum. The others are New World spiders:
Anelosimus eximus (and possibly a second
species of the genus, A. studiosus) in South
America and one of the species I have col-
lected in Mexico, Mallos gregalis. All have
in common the habit of constructing a large
central web that is occupied by all the spi-
ders in the aggregation. By combining their
labors the spiders are able to construct a
web that is much larger and far more elabo-
rate in architecture than the web of any
single spider; the structure is occupied by
successive generations.
These spiders also collaborate in captur-
ing prey much larger than prey any one of
them could capture alone. Moreover, after
the prey has been captured the spiders feed
on it communally. Interactions as .complex
as these imply that these species have in
addition to a tolerance mechanism a capaci-
ty for the coordination of individual respon-
ses to stimuli and an ability to recognize
intraspecies sensory cues or to respond to
some other kind of information. As an ex-
ample, each spider seems to be able to dis-
tinguish between the web vibrations caused
by a fellow member of the community and
||e vibrations caused by potential prey.
Bertrand Krafft of the University of Nan-
cy has observed Agelena consociata in Ga-
bon. He found that close-quarters tolerance
in the species is mediated at least in part by
chemotactic cues. Uninjured members of
the community tolerate one another. An
injured spider, or one whose normal superfi-
cial odor has been artificially altered by a
washing in alcohol and ether, is attacked
immediately. Neither the chemotactic cues
nor other possible but still unidentified
components of the spiders’ tolerance mech-
anism are confined to local populations of
the species. As with Metepeira spinipes, in-
dividual spiders of the same species can be
moved from one colony to another without
disrupting the communal activity pattern.
There is no evidence that any of these
spider species has evolved a caste system
such that the adults differ in form in accord-
ance with any division of labor. Some differ-
ence in behavioral roles may exist as a result
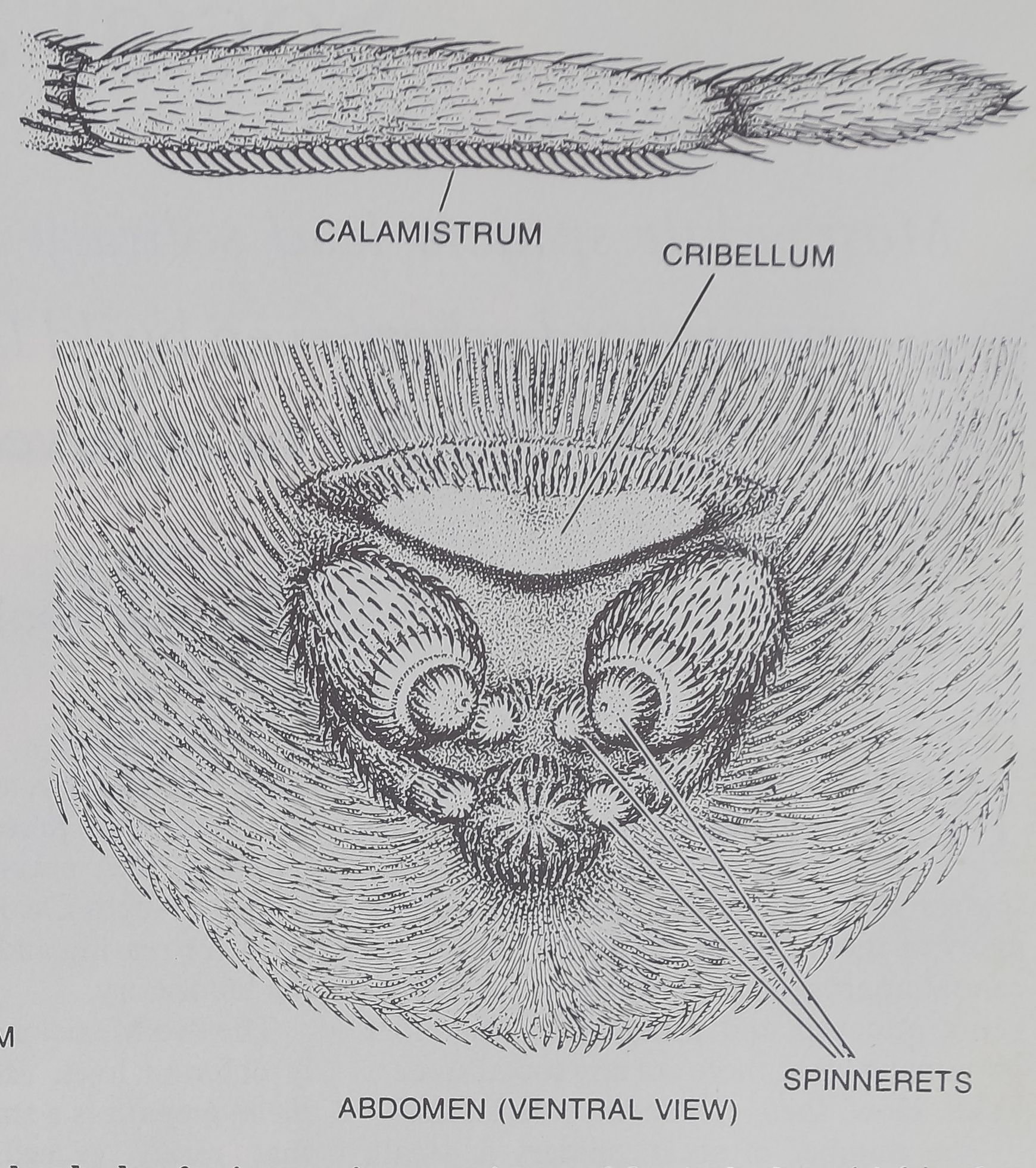
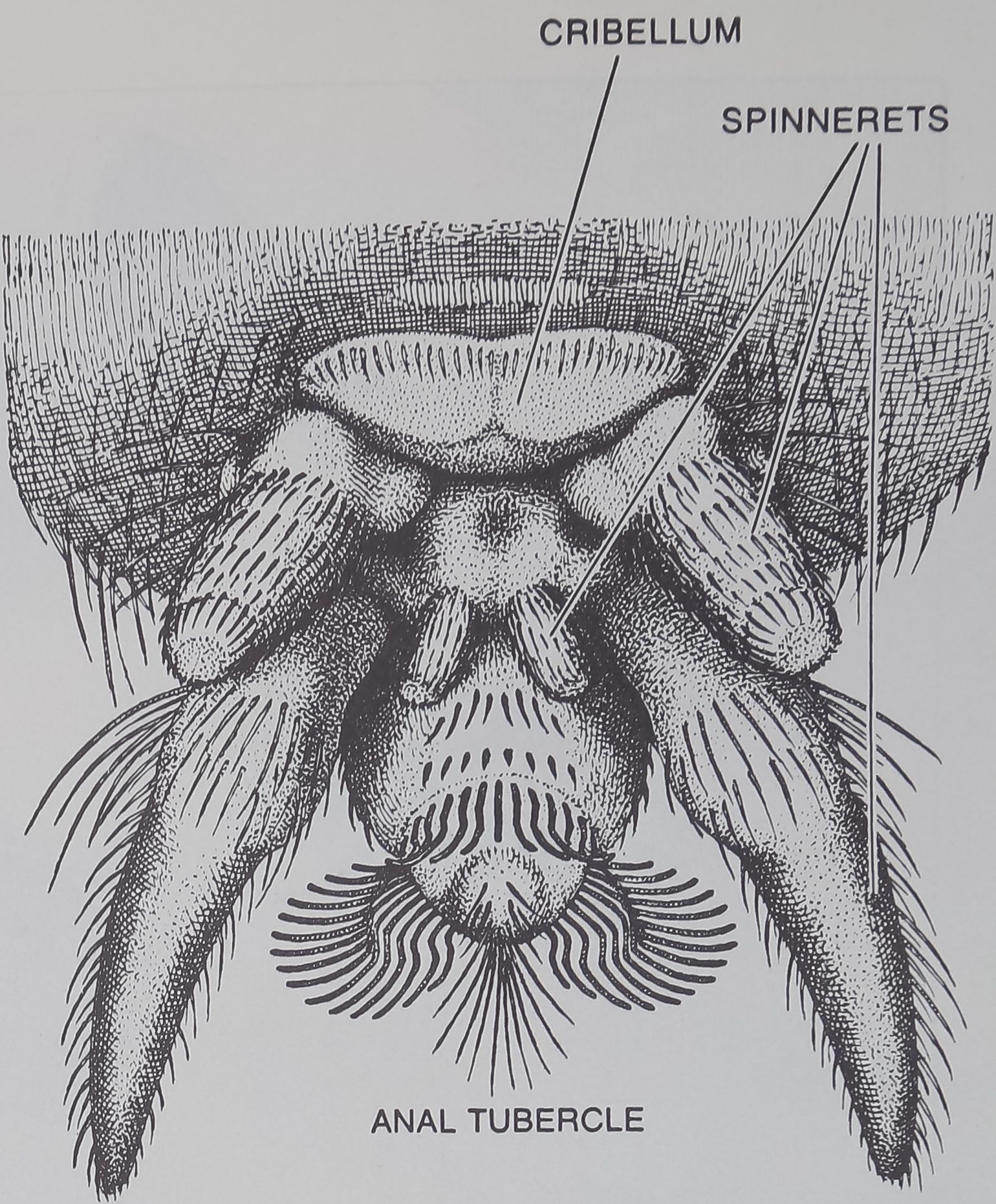
ABDOMEN (VENTRAL VIEW)
GREGARIOUS SPIDER Oecobius civitas has a body averaging two waiting for it to become entangled. The spider combs out a thread of
and a half millimeters in length. Like Mallos gregalis it has a cribel- the silk with its anal tubercle (right) and winds silk around the prey
lum, but it uses its sticky Hk actively, wrapping ils prey rather than by circling it, abdomen foremost (see middle illustration on page 106).
of age or variations in biological rhythms,
but just how cooperation is cued remains
unknown. The pattern of behavior is none-
theless an example of sociality that is not
easily equated with any pattern of sociality
among insects. These spiders’ behavior may
Well deserve a category of its own: commu-
nal-cooperative.
The Mexican social spider Mallos grega-
lis traps mostly flies on the sticky sur-
face of the communal sheet web it spins
around the branch of a tree. The spider has
long been known to Mexicans as el mosquero,
the fly-killer, and in the rainy season, when
houseflies are particularly oppressive, those
who live in the Guadalajara countryside
will bring a web-covered branch into their
house in much the same way that other
people might string up flypaper. A member
of the family Dictynidae, M. gregalis is a
cribellate spider. Such spiders have a sieve-
like plate, the cribellum, on the underside of
their abdomen [see illustration on opposite
page]. Sticky silk emerges from fine holes in
the cribellum and is combed away with the
two hind legs that bear a special row of
bristles known as the calamistrum. This is
the silk that forms the sticky prey-trapping
areas on the outside of the spider’s web. The
web as a whole is an elaborate structure that
includes supporting lines running between
the surface sheet and the twigs and leaves of
the branch, sheltered retreats for the spiders
and special chambers where the female spi-
ders live with their egg sacs. The sacs, thin
wrappers of silk, contain from 10 to 20 eggs.
The surface sheet is perforated in places
with holes that provide access to the inte-
rior of the web.
The communal web of M. gregalis can be
very large. One I saw near Guadalajara cov-
ered the limbs and branches in the upper
three-quarters of a 60-foot tree of the mimo-
sa family. Where the limbs met the trunk
the silk of the sheet web was gray, but near
the tips of the branches the silk was new and
white. Evidently construction was continu-
ing outward along the limbs. The spiders
were not confined to the newer portions but
were active in all parts of the web.
Both field and laboratory observations
confirm that the construction of the Af. gre-
galis web is a mutual effort. If a laboratory
colony of the spiders has some treelike sup-
port available, such as an upright stick, the
spiders will build their characteristic envel-
oping sheet web. In the absence of such a
support they will build the kind of three-di-
mensional web that is typical of other dic-
tynid species. Although this web looks dif-
ferent from the natural one, it too includes
retreats and egg-sac chambers. In the labo-
ratory web a task begun by one spider may
be finished by another. I have also seen one
spider of the colony lay down strands of
ordinary silk, after which other spiders add-
ed bands of the sticky cribellate silk.
Observed in nature, the spiders seem to
move around at random and without haste,
emerging from and disappearing into the
holes in the surface of the web. Their fly
prey are trapped by the sticky web when
they alight on it. When a fly gets stuck, two
or three spiders approach the buzzing in-
sect, immobilize it with their venomous
bites and then feed on it. On occasion the
spiders can be seen carrying flies down the
holes into^the interior of the web.
The spiders’ predatory behavior can be
observed in detail in the laboratory. We feed
our colonies once every five days, which
increases the probability that a majority of
the spiders will be ready to feed at the same
time. At any given moment one or two indi-
viduals in a colony of about 100 spiders are
usually on the surface of the web; the other
spiders will be in the web’s interior. When a
housefly is put into the spider cage and flies
about, it makes a humming noise that is
audible to the experimenter but causes no
apparent change in the random activity of
the spiders.
A fly that lands on a nonsticky part of the
web and walks around stimulates a local-
ized response; some of the spiders will turn
to face in the direction of the fly, but that is
all. If the fly gets entangled in a sticky part
of the web and begins to buzz loudly, the
behavior of the spiders changes abruptly.
Throughout the web spiders that have been
at rest turn toward the trapped fly and begin
to approach it in short jumps. The fly con-
tinues to buzz even after the first spiders to
reach it start their attack, usually by biting a
leg or a wing. The buzzing draws more at-
tackers; they move directly toward the fly
over the web surface until eventually the
prey almost disappears under the feeding
spiders. Both male and female spiders at-
tack. Even immature spiders take part,
swarming over the adults in search of a
place to feed.
Even though the attacking spiders in the
caged colony are quite aggressive, we have
never observed one spider attacking anoth-
er. As we canvass the behavioral repertory
that differentiates social spiders from soli-
tary ones, this aspect of feeding in aggrega-
tions is significant. For example, young soli-
RESPONSE TO AN INTRUDER by a colony of communal spiders
is reconstructed in these drawings on the basis of. laboratory observa-
tion. Unlike the members of a wild colony all the spiders in the labo-
ratory colony have fasted the same length of time. The sound of a
passing fly is audible to a human observer but attracts no attention
from the spiders. Even when a fly lands on the surface of the web (top
left), only the nearby spiders reorient themselves. The buzzing of a
fly entangled in sticky silk (top right) stimulates a response through-
out the colony, and the spiders advance on the prey in quick jumps.
The bites of the first spiders to reach the fly (bottom left) give rise to
a louder buzzing that further stimulates spiders to approach the prey.
As feeding begins (bottom right) immature spiders join the adults.
WEB-COVERED BRANCHES of a species of mimosa near Guadalajara support part of the
communal web of a Mallos gregalis colony. Scattered holes allow the spiders to move freely
from areas inside the web to the sticky outer surface where intruders become trapped. In the fiy
season local people often bring such branches indoors to serve as a kind of natural flypaper.
tary spiders such as those of the species
Araneus diadematus, when they are artifi-
cially confined in close quarters, will also
feed communally. Among the artificially
confined solitary spiderlings, however, a
tolerance mechanism, if it exists at all, oper-
ates only imperfectly; they will feed com-
munally both on captured flies and on one
another. This suggests that it is a strong
tolerance mechanism that accounts for
communal feeding in Mallos gregalis just as
coordination mechanisms account for com-
munal capture of prey.
The tolerance mechanism at work in M.
gregalis colonies is being studied in our lab-
oratory. It is evident from our observations
that the mechanism is strong and that it
operates both at close quarters and over
considerable distances. Indeed, several sep-
arate mechanisms may be at work, perhaps
mediated by cuing systems that allow dis-
crimination between, say, the web vibra-
tions caused by trapped prey and those
caused by members of the colony. To test
this hypothesis we are subjecting the colo-
nies to the stimuli of various web vibrations
in the hope of isolating such cues.
The social behavior of the second Mexi-
can spider, Oecobius civitas, at first
seems to be principally aggregative, like the
behavior of other spiders that build their
nests in close proximity. The darkness of
this spider’s microhabitat makes observa-
tion of its behavior difficult, but its unusual
method of prey capture has been recorded.
O. civitas has a finger-shaped organ, the
anal tubercle, on the abdomen near its silk-
extruding spinnerets. With this appendage
it can comb sticky silk out of its cribellum in
a rope that it winds around its prey [see
illustration on page 103].
A closer study of the sociality of O. civitas
proves that it is more than merely aggrega-
tive. The spiders’ behavior features a curi-
ous combination of tolerance and avoid-
ance. On the underside #f the rock that
shelters the spiders each individual weaves
a small open-ended tube of silk that is its
hiding place; around this retreat the spider
constructs a thin, encircling alarm-system
net close to the surface of the rock. The pair
of structures makes up the spider’s web,
which is generally found in a hollow or a
crevice of the rock. If a spider is disturbed
and driven out of its retreat, it darts across
the rock and, in the absence of a vacant
crevice to hide in, may seek refuge in the
hiding place of another spider of the same
species. If the other spider is in residence
when the intruder enters, it does not attack
but darts out and seeks a new refuge of its
own. Thus once the first spider is disturbed
the process of sequential displacement from
web to web may continue for several sec-
onds, often causing a majority of the spiders
in the aggregation to shift from their home
refuge to an alien one.
Field observations and experiments indi-
cate that, as with Metepeira and Mallos, the
mechanisms responsible for the combina-
tion of tolerance and avoidance extend be-
yond the local population to include other
spiders of the same species. Moreover, with-
in the local population the shift to another
spider’s shelter may be a semipermanent
move. The reason is that when the spiders
are undisturbed, they occupy a fixed web
position for long periods. In any event the
behavioral pattern of the species benefits the
individual spider by providing more than
one available retreat in an emergency.
The group behavior of Oecobius civitas is
far simpler than that of Mallos gregalis. It is
nonetheless effective in enabling the spiders
to live together under crowded conditions.
No doubt the avoidance mechanism makes
a major contribution toward the spiders’
ability to maintain a high population densi-
ty in their restricted microhabitat. Other
contributing factors probably include the
spider’s unusual predatory technique and
the spacing of individual webs. In any case,
although we remain largely ignorant of the
mechanisms underlying avoidance and tol-
erance, they appear to be the basic building
blocks that provide a foundation for more
complex group behavior.
It has been suggested that Oecobius civi-
tas displays an even more remarkable kind
of sociality: construction of a communal
egg sac by the females in the aggregation.
The possibility of such a behavioral ad-
vance, unknown among spiders, came to
light recently when William A. Shear of
Hampton-Sydney College undertook a tax-
onomic review of the oecobiid spiders. He
was assisted by a number of colleagues who
donated specimens to the project. Among
the donors was Willis J. Gertsch, curator
emeritus of spiders at the American Muse-
um of Natural History, who had collected
specimens of O. civitas, its web and its egg
sacs in the Guadalajara area.
The usual oecobiid egg sac contains from
five to 10 eggs. In the preserved material
donated by Gertsch, however, Shear found
two groups of more than 200 immature spi-
ders. Each group was contained in what
gave every appearance of being a single
egg sac. Shear published his observation in
1970, suggesting that O. civitas might be a
communal egg layer.
When I collected specimens of O. civitas
and its egg sacs in the area near the shores of
Lake Sayula, where Gertsch had done his
collecting, I found that several other species
of spiders shared the rocky habitat with the
oecobiids. As a result a variety of egg sacs
could be collected. This I did, sealing indi-
vidual egg sacs in individual tubes. I was
disappointed to find that only the small
sacs, averaging seven eggs to a sac and
mainly collected in or near 0. civitas web
retreats, hatched oecobiids.
After rearing this spider in the laboratory
for three generations and observing only
individual egg sacs containing from five to
10 eggs, I consider that to be the normal
pattern of reproductive behavior in O. ci-
vitas. To resolve the question beyond all
doubt other single 0. civitas egg sacs con-
taining eggs or immature spiders in large
numbers will have to be collected in the
field.
Mating behavior has not yet been ob-
served in our laboratory populations
of either Mallos gregalis or Oecobius civitas.
Solitary male spiders go through elaborate
pre-mating maneuvers, so-called courtship
patterns that supposedly inhibit predation
in the female at the time of copulation.
Among social spiders, which live in tolerant
aggregations, such maneuvers would not
seem necessary. Indeed, if differences in
copulatory patterns between solitary and
social spiders do exist, they may even pro-
vide clues to the evolutionary background
of spider sociality. In this connection we
have made one possibly significant observa-
tion concerning fertility. Solitary spiders
raised in the laboratory retain the cyclical
breeding rhythms characteristic of their
wild state, but when our M. gregalis colo-
nies are provided with a uniform environ-
ment and controlled periods of darkness
and light, they produce fertile eggs through-
out the year.
Observation of the two Mexican spiders
has uncovered a substantial amount of in-
formation about their sociality, but that
information more often than not merely de-
fines the extent of our ignorance. For exam-
ple, we do not know what conditions favor
the development of spider sociality or even
what mechanisms are involved in tolerance,
avoidance, the formation of groups or the
coordination of activity. Moreover, it is not
known how different forms of spider sociali-
ty are related to one another or how, in
complex interactions, intragroup informa-
tion is transferred. The search for answers
nonetheless seems to offer one certainty:
The more we learn about the sociality of
comparatively simple animals, the better we
shall be able to understand the sociality of
more complex species, including our Own.
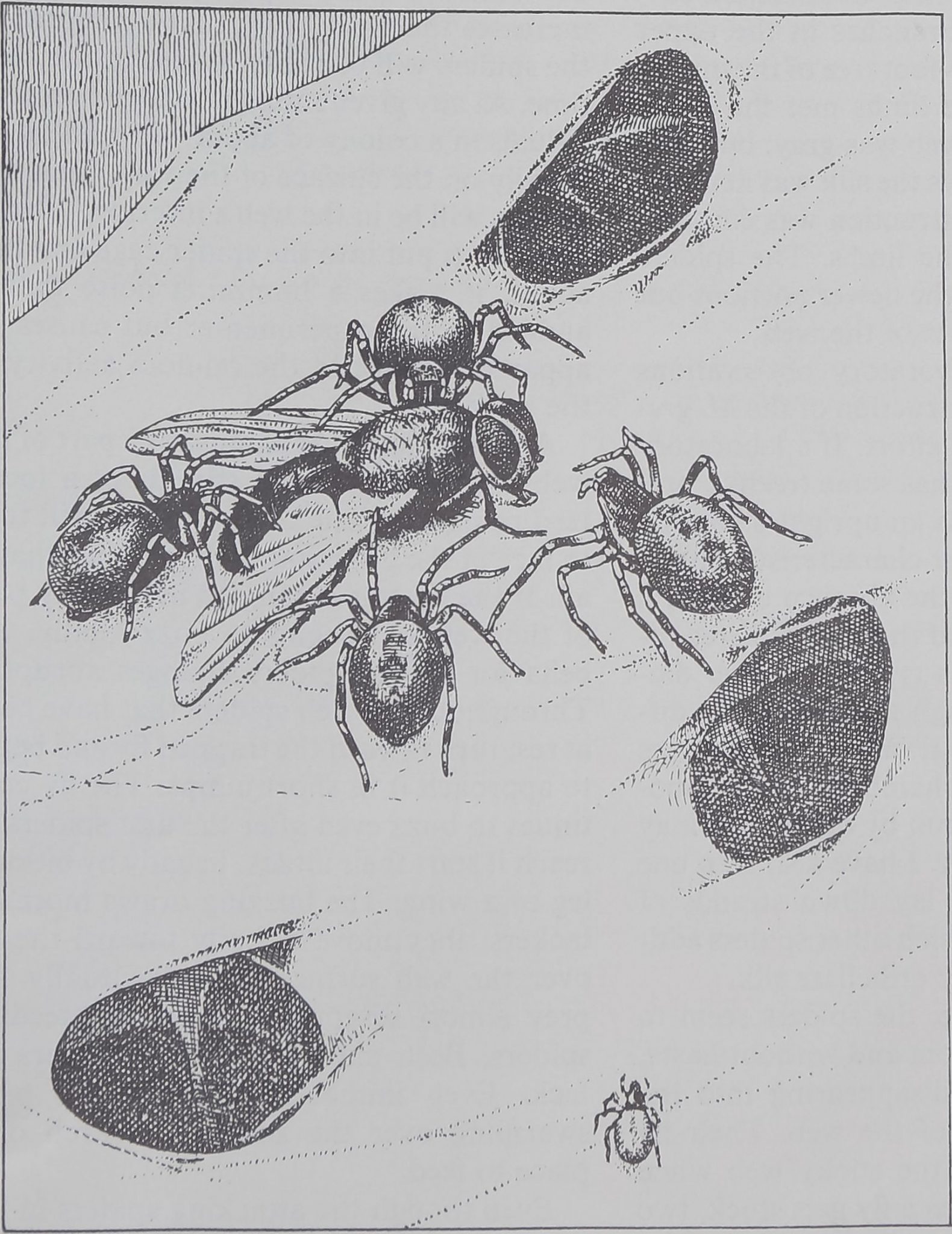
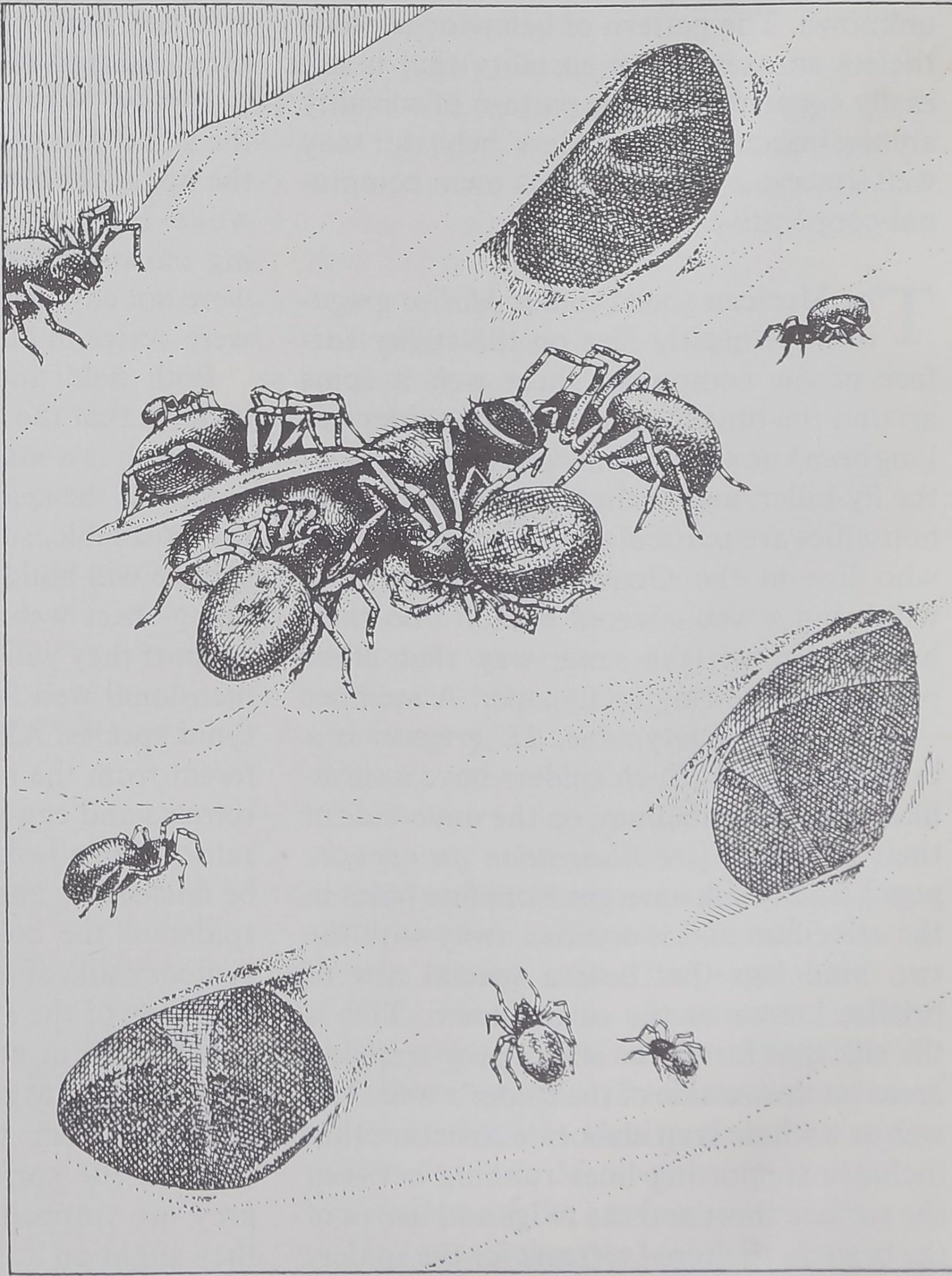
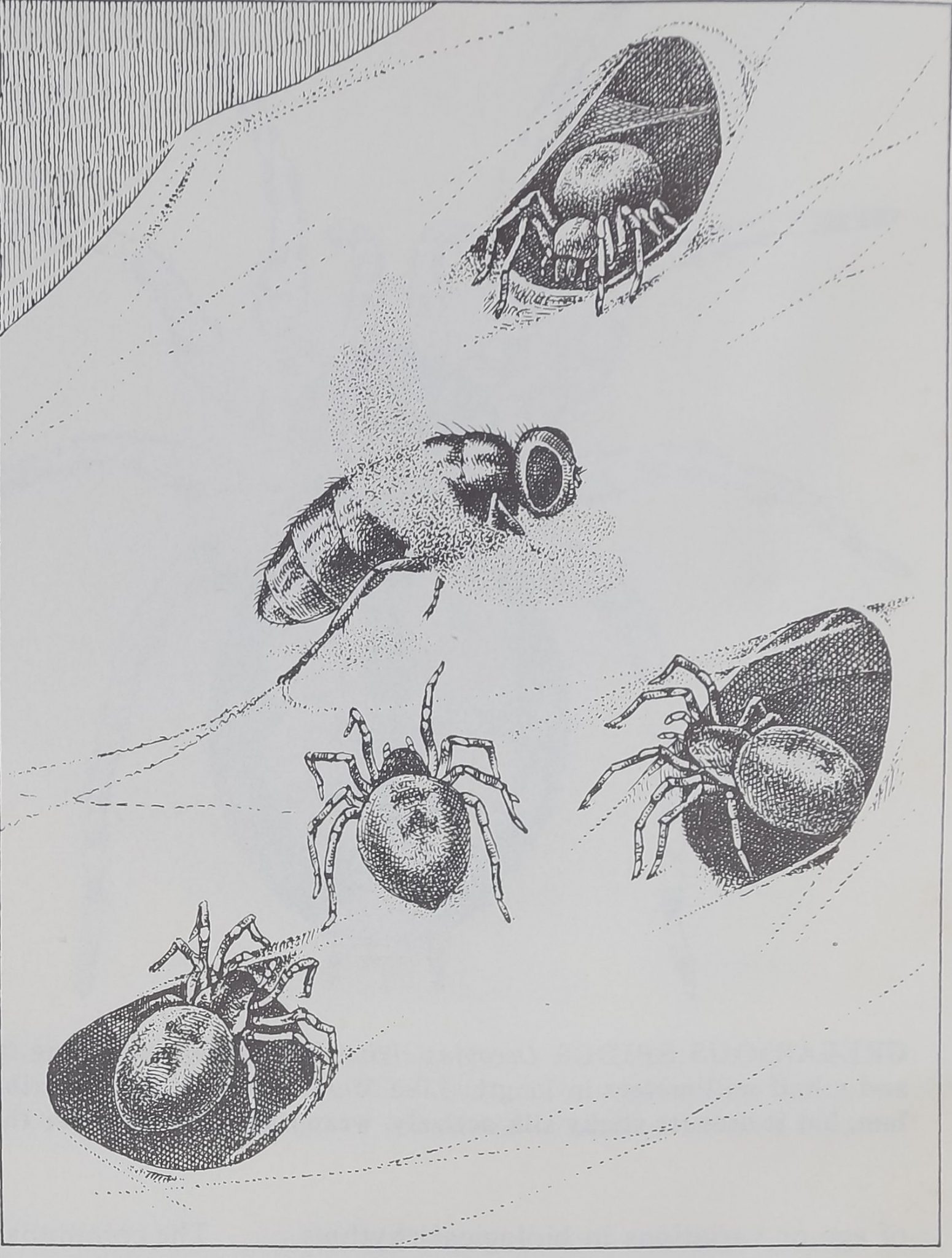
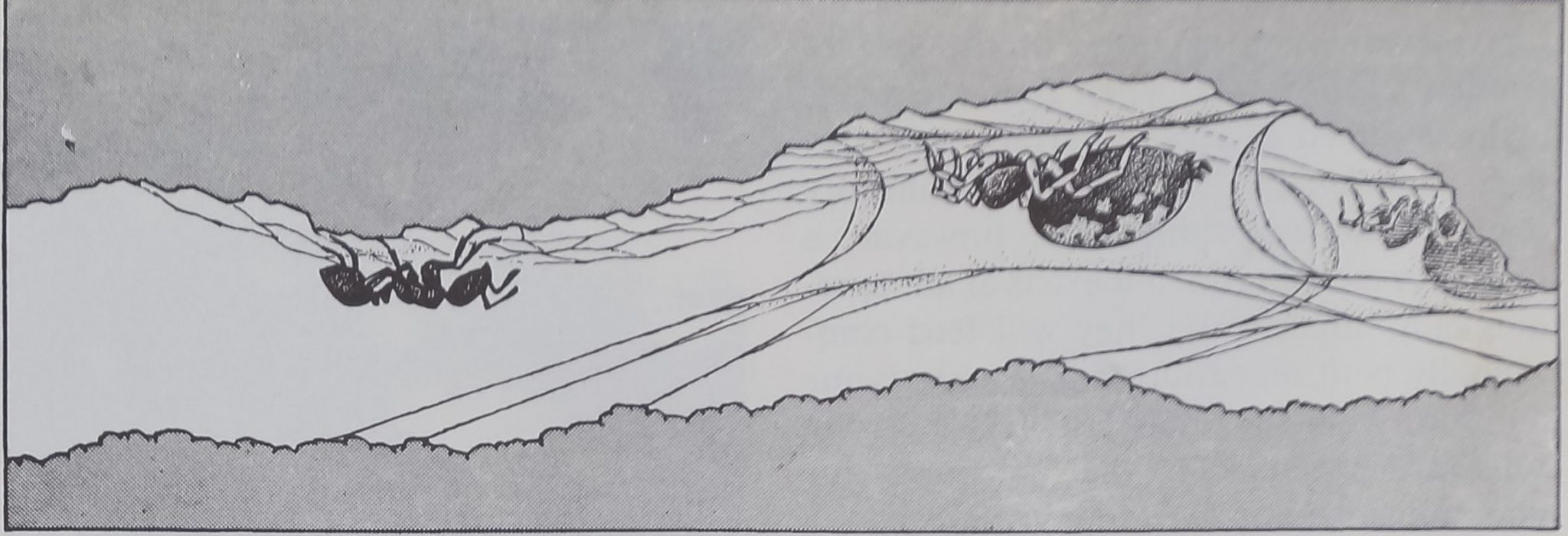
CAPTURE OF PREY, usually a foraging ant, by a spider of the gregarious species Oecobius ci-
vitas follows a complex pattern that begins when the intruder disturbs the spider’s alarm web.
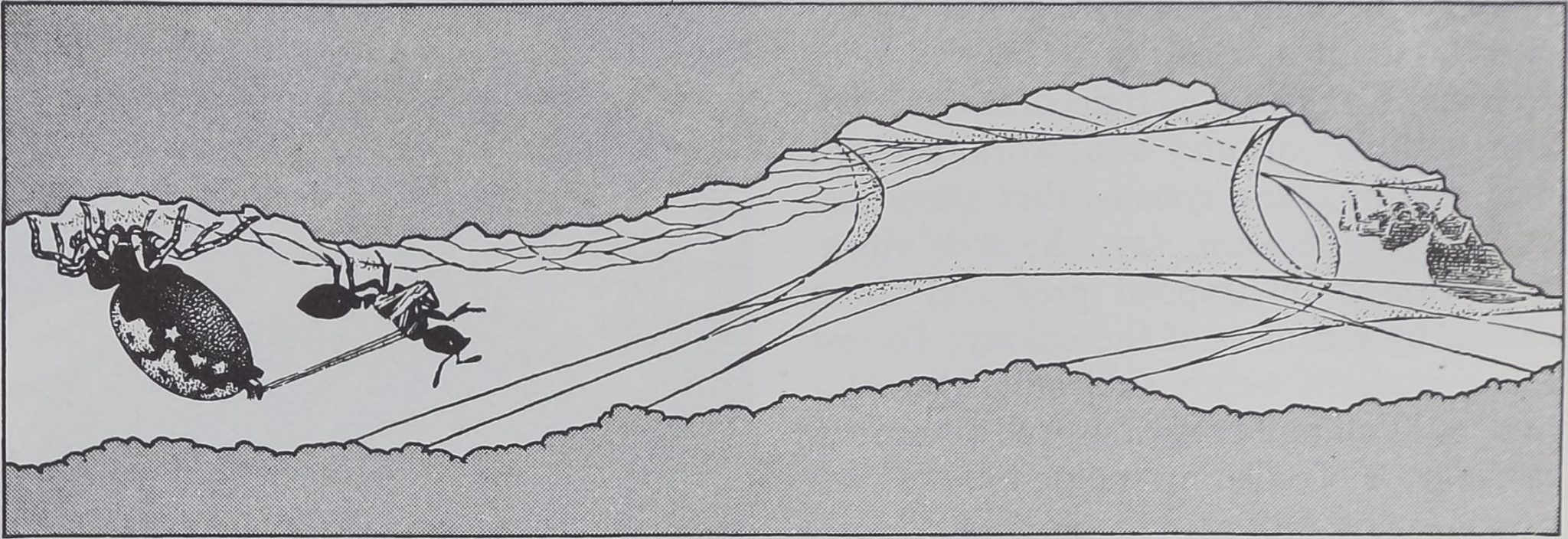
ALARMED SPIDER leaves its shelter and moves in circles around its prey, its abdomen fore-
most and raised clear of its legs, while it combs out a strand of sticky silk with anal tubercle.
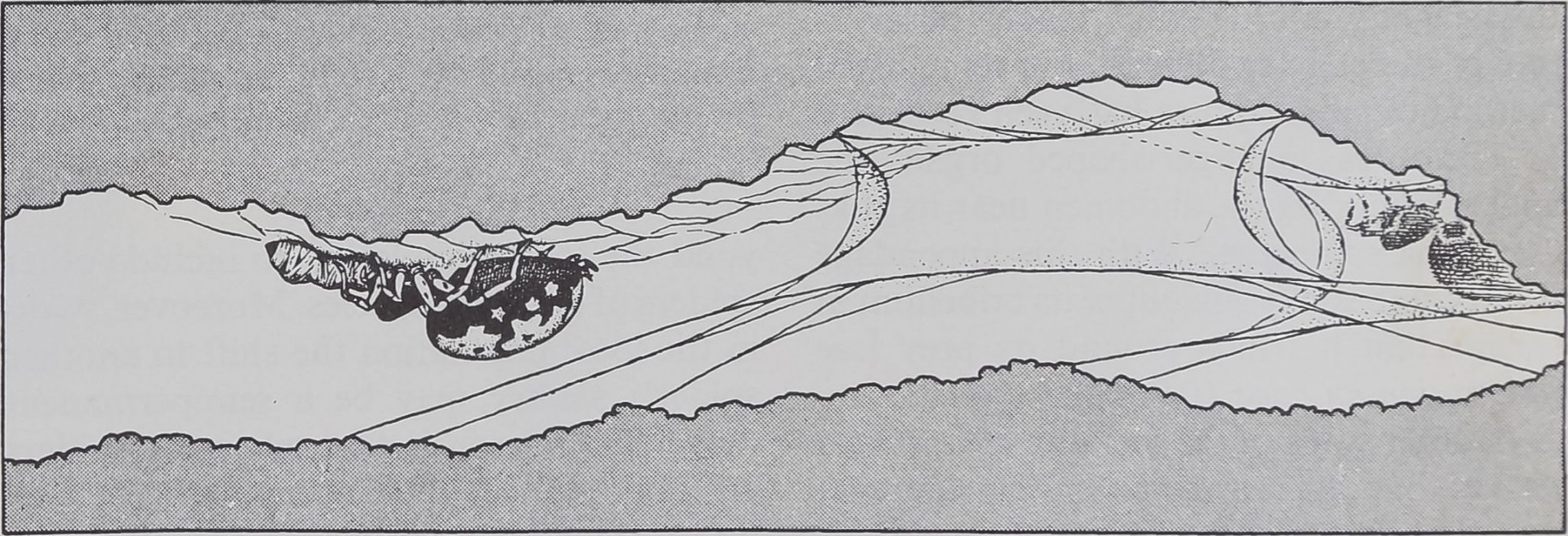
WRAPPED IN SILK, the ant is immobilized. The spider may rest for a time or may turn (left)
to bite and disable its prey. Only the captor feeds on the prey; nearby spiders do not approach.