Images Collection
Read OCR Digitized Article Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
Reprinted from Tun Journal of Pharmacology and Experimental Therapeutics
Vol. 112, No. 4, December, 1054 Printed in U.S.A.
ACTION OF K-STROPHANTHIN ON POTASSIUM LEAKAGE FROM
FROG SARTORIUS MUSCLE
HANS JÜRG SCHATZMANN and PETER N. WITT Department of Pharmacology, University of Berne, Switzerland
Received for publication September 7, 1954
Several authors have pointed out that cardiac glycosides enhance the leakage of potassium from the cell, both in active heart and skeletal muscle (Cattell, 1938; Cattell and Goodell, 1937; Guttman and Cattell, 1940; Hagen, 1939; Orabona and Manganelli, 1954; Sherrod, 1947; Wood and Moe, 1938). In the present paper we are trying to differentiate the effect of Strophanthin on potassium leakage in active and resting muscle. We want to answer the question whether the drug predominantly acts during the resting phase or during the action potential, i.e. whether it impedesthe active inward transport of potassium during rest or facilitates the breakdown of the cation barrier during excitation. By answering this question we hope to elucidate the problem of the site and mechanism of action of cardiac glycosides in the muscle cell.
Strophanthin in resting cells seems to influence the active sodium-potassium transport: one of us (Schatzmann, 1953) was able to show that cardiac glycosides apparently block the sodium-potassium transport in red blood cells which normally maintains the characteristic salt distribution on either side of the membrane. If comparison between erythrocytes and resting muscle is justified as regards the action of digitalis, the blocking of active cation transport in resting muscle might be considered the cause of potassium leakage observed; the same could hold true for contracting muscle. This view is held by Hajdu and Szent-Györgyi (Hajdu, 1953; Szent-Györgyi, 1953).
‘ By poisoning resting and active muscles with 2,4 dinitrophenol (DNP) we hoped to’ obtain information about events during activity, as this substance blocks the active cation transport in the resting cell (Hodgkin and Keynes, 1954; Maizels, 1951). It is not improbable that it has the same effect on skeletal muscle cell, and a Strophanthin effect still apparent under DNP in active muscle could be attributed to interference with a mechanism different from the active cation transport during the resting phase. However, it has to be taken into consideration that we only made overall estimates of potassium movements over a long period of time. This procedure will be analyzed in detail in the discussion.
Methods. Frog sartorius muscles were dissected, impairing as few fibers as possible. Around both ends threads were tied by means of which the distal ends were fastened to glass hooks, the proximal ends to 2 gram weights which stretched the muscles vertically at a standard tension. With its lower, distal three quarters each muscle dipped into a Ringer bath which was continually stirred by bubbling air.
After having been prepared in normal Ringer the muscles were washed immediately preceding each experiment with potassium-free Ringer’s solution. Then they were suspended in 4 to 6 ml. of test solution: 20 minutes in experiments with resting muscle, 10 minutes in experiments with stimulated muscle. Then each bathing fluid was collected in a test tube for
501
SCHATZMANN AND WITT

potassium measurement, and the same volume of fresh solution of equal composition put into the bathing vessel. This was repeated at least 4 times per experiment.
The Ringer used was Nastuk and Hodgkin’s “Normal Ringer” (Nastuk and Hodgkin, 1950); potassium chloride, however, was left out altogether. Samples of the Ringer were measured before and after contact with the muscles, and the potassium values of the blank subtracted from those found after the muscle had been in the solution. The 10-B Strophanthin solution was prepared from 100 ml. of the above-mentioned potassium free Ringer by adding 2 ampules of “Strophoside forte Sandoz” (containing 0.5 milligrams each). Two-tenths millimolar DNP solution was obtained by dissolving 3.68 milligrams of DNP in 100 ml. of potassium free Ringer.
In several experiments rhythmic stimuli were applied as condensor discharges, one platinum electrode touching the muscle in air, the other one touching the muscle at the bottom of the bath. The muscle was fastened to an isometric lever. The size of stimuli varied, the voltage being chosen so as to get just maximal response; this frequently meant an increase in voltage in the course of an experiment. As one pair of muscles was stimulated in one circuit, the stimulus for both muscles was of the same size. In our short experimental time (40 minutes) no constant difference between treated and untreated muscle in regard to activity could be noted.
Potassium content of each sample was measured in a flame photometer. After subtracting the potassium content of the blank the potassium that had been lost from the muscle into the bathing solution was expressed in micromols (mM) per 10 minutes. In comparing a treated and an untreated muscle of the same frog the weight was of no account and was therefore not taken into consideration.
The evaluation of the experiments had to be done statistically, as the values varied widely from muscle to muscle. In a muscle pair the potassium loss of the treated muscle was subtracted from the value for the untreated muscle, resulting in a figure above zero when the drug had enhanced the potassium loss and being zero if there was no effect of the drug on potassium loss at all. The differences of all pairs treated in the same way were pooled, their means compared to zero with Student’s t-test (Linder, 1951).
In order to compare muscles from different animals which had been treated differently an effort was made to consider the weight by expressing the potassium loss per gram muscle. However, nothing was gained by this procedure as the standard error of the means stayed the same, and light and heavy muscles seemed to be about equally distributed over the various groups of experiments. As there is no certainty about the relationship between size of muscle and potassium loss, we finally decided not to introduce a new factor into our calculations and left the weight out. The mean potassium loss per 10 minutes of all muscles treated in the same manner was calculated and compared to the mean of another group with the t-test.
In the experiments with resting muscles the potassium loss during the first twenty minutes was not taken into consideration; the reason for this will be discussed in the following section. In the experiments with twitching muscles all values were used for calculating the potassium loss.
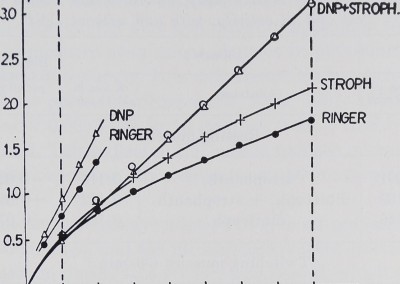
Results. The potassium loss of a whole sartorius muscle (weighing about 200 mgm.) in potassium free Ringer was, under the experimental conditions described above, in 22 experiments, 1.819 /xM in 160 minutes. In the first 20 minutes an average of 0.537 pM was lost, i.e. nearly a third of the total loss. In the following minutes the speed of potassium loss slowed down and was only 0.133 /iM in the last 20 minutes of an experiment. In figure 1, where the average 20-minute values are added up, the total loss into the Ringer, up to any given time, is indicated by the lowest curve, the average 10-minute loss being 0.0913
mM.
K-STROPHANTHIN ON POTASSIUM LEAKAGE
503

Fig. 1. Abscissa: experimental time in minutes. Ordinate: total potassium loss of the muscle from the beginning of the experiment in micromols. Vertical dotted lines indicate those parts of the experiments that were used for evaluation. Each point in the graph represents the means of all experiments done in the same way. The steeply rising lines on the left are of twitching muscles, the three longer ones of resting muscles. Note the higher potassium loss of resting muscles under the influence of Strophanthin as compared to that of untreated muscles. The muscles in DNP and those in DNP plus Strophanthin lose potassium at the same rate.
By adding Strophanthin we were able to increase the potassium loss; this is indicated by a steeper rise of the curve in figure 1; an average loss of 0.1155 pM per 10 minutes was calculated. In nine experiments where the muscle was suspended in Ringer with DNP 0.2 millimolar to block the sodium pump (Hodgkin and Keynes, 1954; Maizels, 1951) even more potassium was lost (an average of 0.1885 pM per 10 minutes). Table 1 shows the average losses which are significantly different below the 0.01 probability level.
If the muscle is suspended in a bath with DNP 0.2 millimolar plus Strophanthin 10_5 there is no difference in potassium loss from DNP alone, as indicated in figure 1 by the circles lying on the triangles. The loss was calculated as an average of 0.1818 pM in 10 minutes. No difference could be shown statistically between the DNP effect with and without Strophanthin in resting muscle (table 1).
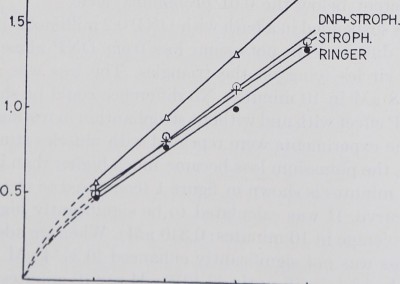
When the same experiments were repeated with muscles stimulated at a rate of 30 per minute, the potassium loss became much higher than in resting muscle. The loss over 40 minutes is shown in figure 1 (compared to rest; full circles) and figure 2 lowest curve. It was calculated to be significantly higher than that in resting muscle (average in 10 minutes: 0.340 pM). When we added Strophanthin the potassium loss was not significantly enhanced (0.3454 pM per 10 minutes). DNP increased the loss significantly (0.4219 pM per 10 minutes). In figure 2 this is shown by the Strophanthin curve which represents 24 experiments and a steeper rise of the DNP curve representing 20 experiments. The statistical difference of their means is shown in table 1.
However, a difference between resting and twitching muscles becomes ap-
504
SCHATZMANN AND WITT
TABLE 1
Comparison of potassium loss of muscle pairs, untreated and treated with Strophanthin, at
rest and in activity, with and without DNP
| Muscle 1 | Muscle 2 | Difference of K Loss in /iM per 10 min. | Probability of the Result Being Due to Mere Chance | ||
| Substance | K loss in /iM/10 min. | Substance | K loss in /xM/10 min. | ||
| Resting muscles | |||||
| 0 | 0.091 | strophanth. | 0.116 | -0.025 | 0.2% |
| dinitroph. | 0.189 | dinitroph. + strophanth. | 0.182 | +0.007 | 58% |
| strophanth. | 0.116 | dinitroph. | 0.189 | -0.073 | below 0.02% |
Twitching muscles (30/min.)
| 0 | 0.340 | strophanth. | 0.345 | -0.005 | 80% |
| dinitroph. | 0.422 | dinitroph. + strophanth. | 0.353 | +0.069 | 0.22% |
| strophanth. | 0.345 | dinitroph. | 0.422 | -0.077 | 0.15% |
parent when DNP and Strophanthin are added simultaneously: the potassium loss of a DNP muscle treated with Strophanthin was significantly (beyond the 0.01 probability level) lower (0.3533 juM) than the potassium loss of a muscle in DNP alone if the muscle was twitching (20 experiments). On the other hand no difference could be shown between the muscle in Strophanthin and the one in Strophanthin plus DNP.
In addition no statistical difference in potassium loss between the untreated twitching muscle and the one in Strophanthin could be proved. This may be due

Fig. 2. Abscissa and ordinate as in fieure 1 hut not» j:«? „ • , .,
. , . , „ “Kuie i, oui note the difference in scale. All muscles
were stimulated a a rate of 30 per minute. Only the muscles in DNP showed a significantly higher potass,urn loss than the untreated ones. Strophanthin plus DNP make the muscles lose less potassium than DNP alone, which is in contrast to the findings in resting muscle
K-STROPHANTHIN ON POTASSIUM LEAKAGE
505
either to there being no difference at all or to an insufficient number of experiments; regarding these values no statements can be made.
Discussion. All experiments were performed in potassium free Ringer solution so as to be able to measure even minor changes in potassium concentration in the bath with sufficient accuracy. It is well known that muscle in potassium free Ringer solution loses potassium (Steinbach, 1940, 1951, 1952). Accordingly we found a mean loss of 0.0913 nM- in 10 minutes from a freshly dissected muscle if no drug was added. This indicated the muscle was not in equilibrium, and care must be taken when applying our results to experiments with muscles in complete Ringer.
The resting muscles were always allowed to give off potassium into potassium free Ringer for 20 minutes, and the measurements were started afterwards. The rapid loss of potassium during this preparatory period, which our curves suggest (figure 1), is most probably due to the intercellular space equilibrating with the bath. This first phase of rapid loss of potassium merges smoothly into a more or less rectilinear course which we assume to represent the rate of potassium loss from the cells.
In twitching muscles this rate is higher, and therefore the first phase of an experiment shows nearly the same rate as the following ones. This led us to include the values measured in the first 10 minutes into the calculation of experiments with twitching muscles.
In the experiments with resting muscles the bathing fluid was exchanged every 20 minutes, in the experiments with twitching muscles every 10 minutes. As the volume of the bath was 20 to 50 times that of the muscle an accumulation of potassium in the bath at the end of this time can be neglected. The concentration in the bath in fact never exceeded 0.9 pM.
DNP was used in a concentration sufficiently high to inhibit oxidative phosphorylation, as was found by Loomis and Lipmann (1948) for various tissues. We added DNP in order to block active transport of cations through the membrane (Hodgkin and Keynes, 1954; Maizels, 1951). The significantly higher potassium loss observed by us in muscles poisoned with DNP seemed proof for its blocking action in our experiments. At the end of an experiment the poisoned muscle developed a maximal response of short tension rise but relatively low maximal tension. However, when the poisoned muscle was subjected to rhythmical stimulation over a period of time it showed rapid deterioration. The higher potassium loss in DNP poisoning observed simultaneously may be due to the fact that the unknown cation transport mechanism is dependent on a supply of energy which is derived from phosphorylation processes and is cut off by DNP.
In the first place our result merely confirms the well established fact that active muscle releases more potassium than resting muscle (Fenn, 1937; Fenn and Cobb, 1935; Fenn, Cobb, Manery and Bloor, 1938; Somogyi and Verzar, 1940/41; Wood and Moe, 1939); as was to be expected this statement holds also true for muscle in potassium free Ringer. That Strophanthin enhances the loss of potassium from resting muscle completes our knowledge insofar as measurements have hitherto only been made with active heart and skeletal muscle
SCHATZMANN AND WITT
506
(Cattell, 1938; Catteil and Goodell, 1937; Guttman and Catteil, 1940; Hagen, 1939; Orabona and Manganelli, 1954; Sherrod, 1947; Wood and Moe, 1938). In active muscle (under our experimental conditions of 30 stimuli per minute and Strophanthin 10~5) 24 experiments did not show a significant effect of Strophanthin on potassium loss. However, after the muscle had been poisoned with DNP an effect of Strophanthin became apparent: the potassium loss was no longer enhanced, but was reduced to nearly the value of unpoisoned muscle. This reversal of Strophanthin action opens a way to the understanding of the influence which cardiac glycosides exert on potassium metabolism.
Generally speaking the measured overall movement of potassium in resting muscle is the resultant of two simultaneous fluxes with which potassium moves in opposite directions through the membrane. The net potassium loss which we measured in the muscle bath accordingly is the result of the outward flux being greater than the inward flux. If the activity of the sodium-potassium-pump (which is responsible for the inward transport of potassium) is reduced without simultaneous change in outward leakage, the inward flux of potassium becomes smaller until a new equilibrium is reached. The inside concentration of potassium is now lower than before, and on the outside a net loss can be observed. It is assumed, as a matter of fact, that DNP blocks the sodium-potassium-pump. The functioning of the pump again seems dependent upon energy-rich phosphate bonds. From the fact that Strophanthin has the same effect on resting muscle (regarding potassium loss) as DNP, one might infer that both substances have the same mechanism of action, namely only interference with energy supply to the cation pump. This assumption, however, is not consistent with our findings in active muscle. Here net potassium movement is again the result of the two opposed fluxes—as mentioned earlier—with an additional outward flux which is due to high potassium permeability during the second part of an action potential. Muscles excited with only 30 stimuli per minute can be considered resting during the greater part of time. During the periods of rest, when the muscle is supposedly not different from an inactive muscle, Strophanthin acts in the same way as in resting muscle, i.e. the leakage of potassium is enhanced. If there were no new effect of Strophanthin during activity, the same enhanced potassium leakage as in rest should be observed. We have shown, however, that Strophanthin in DNP poisoned muscle has a different effect in rest and activity: Strophanthin in a twitching muscle has, in the conditions chosen by us, no effect at all; while in DNP poisoned muscle it has a markedly retarding effect on potassium loss. Therefore an additional factor seems to be responsible for the action of Strophanthin during activity. As there is no reason to believe that DNP has an additional effect in activity (i.e. it invariably blocks the sodium-potassium pump in both cases1), we have drawn the following conclusions regarding the action of strophanthin on potassium movement:
I. Strophanthin has an inhibiting effect on the cation transport mechanism in resting muscle.
1 The reason for this is that DNP induces about the same increment of potassium loss in resting muscle (0.082) and in active muscle (0.101) regardless of the much higher total loss in active muscle.
K-STROPHANTHIN ON POTASSIUM LEAKAGE
507
2. Strophanthin slows down the rate of potassium outflow from muscle during the falling phase of the action potential. (An activation of the cation pump during excitation, which would lead to the same result, seems highly improbable.)
3. If assumption 1 and 2 are correct, these two effects of Strophanthin antagonized each other in our experiments with active muscle in Strophanthin alone. As soon as DNP was added and the pump was blocked, the lowering effect of Strophanthin on permeability during excitation became apparent.
Our hypothesis then is that these two actions of Strophanthin have the same point of attack: the drug interferes with the activity of the cation transporting carrier in the pump as well as with the carrier responsible for the high permeability for potassium during the action potential. This hypothesis further suggests the interesting possibility that the carrier mechanism sensitive to Strophanthin, though operating with a great difference in speed in the two cases, has the same chemical basis throughout.
SUMMARY
The potassium loss of frog sartorius muscle into potassium-free Ringer, which is exchanged regularly, was measured with a flame photometer.
The resting muscle loses significantly more potassium when Strophanthin 10“5 is added to the Ringer, and the loss is still higher in DNP 0.2 mM. There is no difference of potassium loss between a muscle in DNP and a muscle in DNP plus Strophanthin.
A muscle twitching at a rate of 30 per minute loses more potassium than the resting muscle. The loss is not significantly increased by Strophanthin 10“5, but is higher in DNP 0.2 mM. Adding Strophanthin to the muscle twitching in DNP reduces the overall potassium loss significantly, when compared to the loss in DNP alone.
Arguments are advanced in favor of the hypothesis that Strophanthin blocks the sodium-potassium-pump in resting muscle, but has an additional retarding effect on potassium outflow during the falling phase of an action potential in the twitching muscle.
REFERENCES
Cattell, M.: This Journal, 62 : 459, 1938.
Cattell, M., and Goodell, H.: Science, 86:106,1937.
Fenn, W. O.: Am. J. Physiol., 120: 675, 1937.
Fenn, W. O., and Cobb, D. M.: Am. J. Physiol., 115: 345, 1935.
Fenn, W. O., Cobb, D. M., Manery, J. F., and Bloor, W. R.: Am. J. Physiol., 121: 595, 1938.
Guttman, S. A., and Cattell, M.: This Journal, 68: 267,1940.
Hagen, P. S.: This Journal, 67: 50, 1939.
Hajdu, S.: Am. J. Physiol., 174: 371, 1953.
Hodgkin, A. L., and Keynes, R. D.: Symposia Soc. Exp. Biol., 4: Cambridge, 1954. Linder, A.: Statistische Methoden, Basel, 1951.
Loomis, W. F., and Lipmann, F.: J. Biol. Chem., 173: 807, 1948.
Maizels, M.: J. Physiol., 112: 59, 1951.
Nastuk, W. L., and Hodgkin, A. L.: J. Cell. Comp. Physiol., 35: 39, 1950.
Ora bona, M. L., and Manganelli, G.: Bollet. Soc. Ital. Biol. Sperim., 30: 10, 1954. Schatzmann, H. J.: Helvet. Physiol. Acta, 11: 346, 1953.
508
SCHATZMANN AND WITT
Sherrod, T. R.: Proc. Soc. Exp. Biol. Med., 65: 89, 1947.
Somoqyi, J. C., and Verzar, F.: Helvet. Med. Acta, 7: Suppl. VI, 81,1940/41.
Steinbach, H. B.: J. Biol. Chem., 133: 695, 1940.
Steinbach, H. B.: Am. J. Physiol., 167: 284, 1951.
Steinbach, H. B.: Proc. Nat. Acad. Sei., 38: 451, 1952.
Szent-Gyorgyi, A.: Chemical Physiology of Contraction in Body and Heart Muscle, New York, 1953.
Wood, E. H., Collins, D. A., and Moe, G. K.: Am. J. Physiol., 126: 657,1939.
Wood, E. H., and Moe, G. K.: Am. J. Physiol., 123: 219, 1938.