Images Collection
View this article in Search Friendly Plain Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
Body, Web-building and Feeding
Characteristics of Males of the
Spider Araneus diadematus
(Araneae: Araneidae)
BY
Reprinted from Psyche, VoI. «0, MC 1-2, March-June 1973
pages 22-+7
BODY, WEB-BUILDING AND FEEDING
CHARACTERISTICS OF MALES OF
THE SPIDER ARAN EUS DIADEMATUS
(ARANEAE : ARANEIDAEf?
By Ramm RAMfgssE* *
D^^K ^I Research
Department pf Mental Health
Box
27611
female orb-web spiders in their
natural’ habitats ( Enders, ‘ 1972; ’ but there have
been relatively few scientific observations of males out|Jt^?s. A major
reason fori :Miler3|lSration males discontinue web-
‘%iildïn^®^^^^;”tee]^y||tés and;4re dl^#t to follow in an un-
àfielÉion in labora-
tory situations since they have shorter females and
The activity
relation to
ÉÉfaeir y.^^BMMing behavior (LeGuelte, 1966, Witt, A§t$3a,b),
making th^^Rpn^a more frequent Thus, with
th^^^M^M||B|^fcfca.ti<e>n al„ 1972),
only Ihav^^^fero&nreheiaife8BWw^i8yro
The of the males
ofBy%«^«MÉBK’SÉA and the female-male
P^^feionshiultimately determines the continuity of the species.
TwoWMli related to the fepaal^^gp; ÿready been identi-
fied as possibly pfcy^Si pa A in the /glWival of the species. These
include and aturing. Cocoons have
been observed hatching at two different times for a single species of
spider — presumably providing an advantageous distribution of egg-
production over a period of time (Potzsch, 1963)» Also, within a
set1 of spiderlings, different rates of maturation have been observed.
Some females grow rapidly and die early while others grow slowly
•Present address of author: Laboratoire d’Ethologie experimentale, 1 rue
Raulin, 69 Lyon 7®, France
Manuscript received by the editor March 1973
*To avoid confusion with the designation of “family” used in nomencla-
ture, offsprings from a single cocoon will be called a “set.”
22
1973] Ratwousse— Araneus diadematus 23
and live at least four months longer (Reed & Witt, 1972). The
related differential maturing rates may provide an advantageous
distribution of spiderlings over a period of time. Together, these
mechanisms would seem to help a species survive drastic or poten-
tially destructive changes in environmental conditions. This research
seeks to explore the male’s role in these phenomena. At what rate is
he growing, maturing and dying during the female’s life cycle?
This leads to the question of inbreeding. An observation of the
maturation rates of spiderlings of the same set was conducted in an
effort to determine if inbreeding is possible.
Also, if the rate of growth is a factor in the rate of maturation
(and spiders of the same set are known to present a considerable
variation in size even under apparently optimal conditions), (Witt
et al.y 1968), is growth prenatally or genetically determined or a
function of external factors?
The effects of an even diet independent of manifest behavior (Witt
et al.y 1972) and differential force-feeding on various schedules
(Benforado & Kistler, 1972) have already been studied. What,
however, would happen to the growth rate of male and female
spiders if they could choose their food quantity through web-building
frequency ?
The answers to some of these questions about the growth and
maturation of male spiders should provide clues about their role in
the reproductive cycle and, more generally, about their role in the
continuity of the species.
METHODS
Two Araneus diadematus cocoons collected in the field, were placed
in two different rearing boxes in the laboratory, where they hatched
(February 23, 1972, one cocoon and 14 days later, March 6, 1972,
the other). The offspring from the first cocoon will be called set I,
and the offspring from the second cocoon, set II. The laboratory
provided a cycle of long warm days and short cool nights throughout
the lifespan of the animals.
As the animals left the communal web to build individual webs,
they were put in glass tubes. Five weeks after hatching for set I and
three weeks after hatching for set II the spiderlings were caged in
individual labeled frames (50 X 50 X 10 cm) where they could
build webs without apparent limitation in size. All observations
began at this moment; however, some molts were noticed inside the
cocoon, and the spiderlings molted one or two times in the glass tubes.
24
Psyche
[March-June
In the rearing boxes as well as in the glass tubes they were pro-
vided water and gnats ad libitum. In the frames the spiderlings were
fed with de-winged ‘houseflies. A weighed fly was given one time
every three days only when a web had been built, thus rewarding the
spiders for high frequency of building.
The individual weights, oif the. spiders (accuracy ô’.’î mg) were
recorded every week and web-building was recorded every day. Each
web was photographed then collapsed 1^ the experimenter, and ana-
lysed fof Structure and regularity (Reed tt al.,
I9és). The dales =®f the molft^f each spW^ylteg were recorded and
the1 length of fKP first lfe‘g : W’as measured’ Pi the” fpiltéd limb (ac-
curacy in mm. ).
In iâtfe Jfcj|j&s FG and SG are used in place
of fast gfâwr^’ Males’ and slow growing males. IIIpootcM ebm-
parlspn hefeiéA jhf’twp.groups (SG & FG) Where not Sp^ffically
mentioned was made with thë WfMék^^%#ti(‘&dapted by White for
Impaired mé^sufrinents (White?;1
Ig/ IJNP |i ‘ t|fji| reached iryVnil* twelve were
identified a® males. There Jj ■ ■ ITijyA’ifif* animals
M set I^^’Thfe ifemhej^ «females’ Uy^fech set is significative repre-
s^itafiivdieif-^6 , expected of males in sc.population
(Binomial test, p = o.oI in each east).
Some characteristics of the male>
The adult males of Æw%n,ms diademhave enlarged blade palps,
relatively n-arslJW ‘elongated abdomens, and weigh about a fif-th of the
adult females. Adult females- are characterized by long yellow palps
and a globulous abdomen ( Figure ÉH^rOthef characteristics of the
males include banding of. the legs that is* generally darker, a lack of
humps on the abdomen, and a modified second tibia that is stronger
than in females and has short spines (Levi, 19^1 )•
The enlarged palps appear at the end of the next-to-the-last molt,
whitish instead of black, and blacken between the two last molts.
One animal exhibited enlarged palps prematurely two molts before
the last one and four other animals after the last molt, but these
were exceptions.
After the last molt, when they reached sexual maturity and maxi-
mum weight, the males stopped building webs. Sekiguchi (1955)
reported that a male of Araneus ventricosus, in the laboratory, did
Ranwusse — A raneus diadematus
25
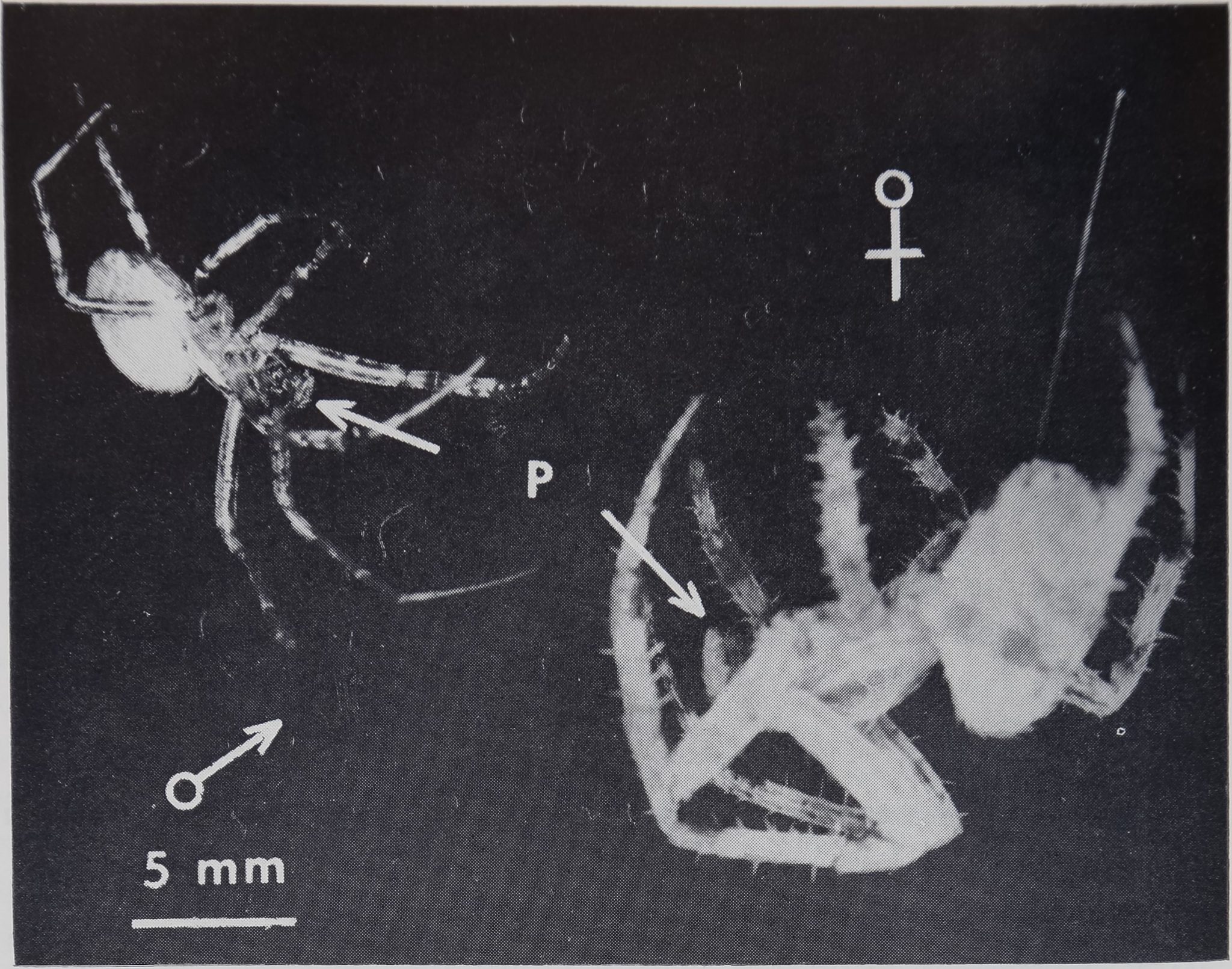
Figure 1. Outline of a male (left) and a female (right). Note the
difference of size (female front leg: 16 mm, male front leg: 12 mm), of
weight (female: 144.1 mg, male: 47.0 mg) and the difference of form of
the palps (short and enlarged for the male, long and thin for the female).
not spin a web after its last molt, and that the aggregate glands
become vestigial in the adult males. Prior to this point the involve-
ment of the aggregate glands in the formation of fhe catching area
of a web was clearly shown (Peakall, 1964). We may suppose that
adult males are unable to spin webs because their aggregate glands
are no longer functional.
The males ate scarcely, even when we attempted to induce prey
catching by placing the flies in front of their mouths. While an
immature male transformed a fly into a small compact ball through
eating; the different parts of the body of a fly abandoned after eating
by a mature male were easily recognizable. Even when they ate, the
mature males used only a small amount of the food available. Males
of Linyphia triangularis Clerck did not require food in the adult
stage, and were still able to mate with females that later produced
fertile eggs. When these males were provided with food, the rate of
prey capture and the rate of food consumption dropped sharply
[March-J une
Psyche
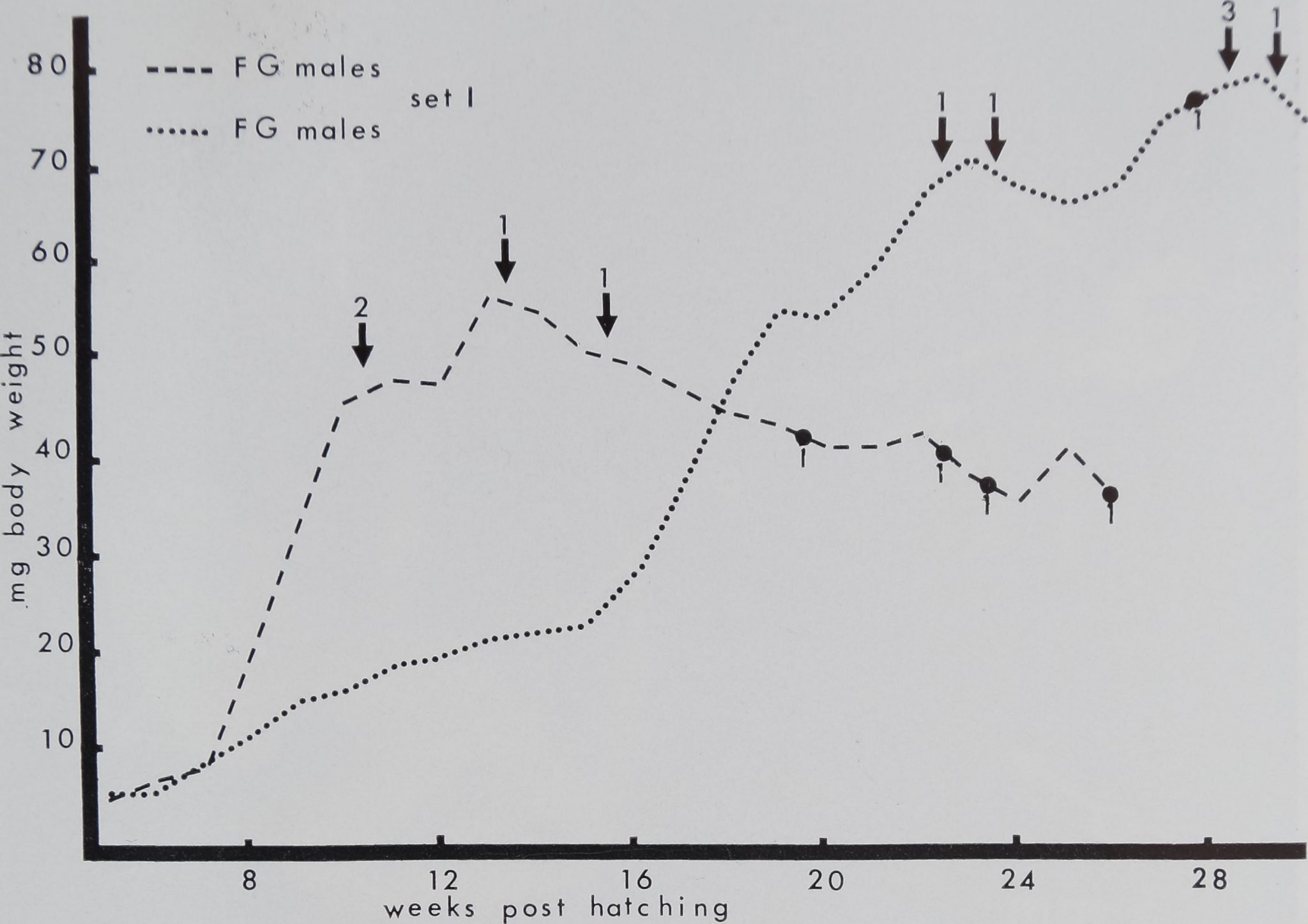
Figure 2. Body weight of four FG and seven SG littermate males in
set I of Araneus diadematus, hatched in the laboratory from one cocoon
on February 23, 1972. Dashed line: weekly mean body weights of the FG
males. Dotted line: weekly mean body weights of the SG males. Numerals
followed by an arrow indicate the number of animals molting for the last
time during a week. Numerals surmounted on black circles indicate the
number of animals dying during a week. The FG males reached their
maximum weight the 13th week of post-hatching, the SG males reached
their maximum the 29th week of~post-hatching. Note that the SG animals
need twice as much time to mature as the FG.
(Turnbull, 1962). We may assume that the adult males, no longer
able to build a web, do not neeed food to fulfill their mating role.
Four males in this study continued to spin webs until they died;
they built webs for a few days after the last molt was recorded, then
stopped building for three or four weeks and generally built a final
web six or seven days before death. These facts suggest that these
four males were not able to go through an additional molt to com-
plete their development. Also, these males presented enlarged palps
only after the last molt recorded which is another confirmation of
thir inability to complete their development.
During the web building period the males are distinct from the
females only between the two last molts (about 3 weeks). This
explains why few studies have been made of the males either outdoors
or in the laboratory.
Ramousse — A raneus
27
If eight increase
In each set, the individual weight curves follow two distinct pat-
terns and no in between: a group with an early maximum (FG)
and a group with a late maximum (SG). In set I the course of the
growth of four males with early maxima (between 10th and 15th
week post hatching) was compared to seven males with late maxima
(between 22nd and 33rd week post hatching) (Figure 2). In the
second set the growth of 11 males which reached their maximum
weight between the 8th and 16th week post hatching was compared
to three males reaching their maximum weight between the 19th
and 23rd week post hatching (Figure 4). In both sets the SG
animals needed approximately twice as much time to complete the
last molt and to attain sexual maturity as did the FG animals. In
each set the females could be divided into fast and slow growth
groups in the same way as males. Figure 6 shows the body weight of
the FG and SG males and females. The data from the two sets
were combined forming four groups : FG and SG males and females.
The weight gain per day until maturation, in both sets, was sig-
nificantly higher for the FG males than for the SG males (set I:
T 6,P||j 0.05; set II: T = 7, P = 0.05).
The mean weight gain per day between the two last molts for
each group was :
set I set II
FG 2.09 mg/d i-59 mg/d
SG 0.64 mg/d 1.61 mg/d
In each set, every animal showed a weight gain per day significantly
higher between the two last molts than during the preceding period
of observation (Wilcoxon matched-pairs signed ranks test: set I:
N = 9, T = 3, P = 0.02; set II: N = 13, T — o, P = 0.01).
Frequency of building
The mean of webs built per day to reach the last molt were:
set I set II
FG 0.57 web/day 0.49 web/day
SG 0.22 web/day 0.18 web/day
28
Psyche
[March-June
The FG males had a higher rate of building while they grew
than did the SG males (set I: T = 6, P — 0.05; set II: T = 6,
P = 0.01). The differences in the rate of building appear clearly
on the graphs (Figs. 3 and 5) obtained by plotting the mean fre-
quency of building per week for each group in each set.
The frequency of building is strongly correlated with the amount
of food eaten per day (Kendall rank coefficient; set I: y = 0.59,
P = 0.004; set II: y = 0.52, P = 0.005). This is the necessary
consequence of the feeding schedule. We might suppose that this
relation occurs in nature. A fresh snare probably increases the
chances of capturing prey.
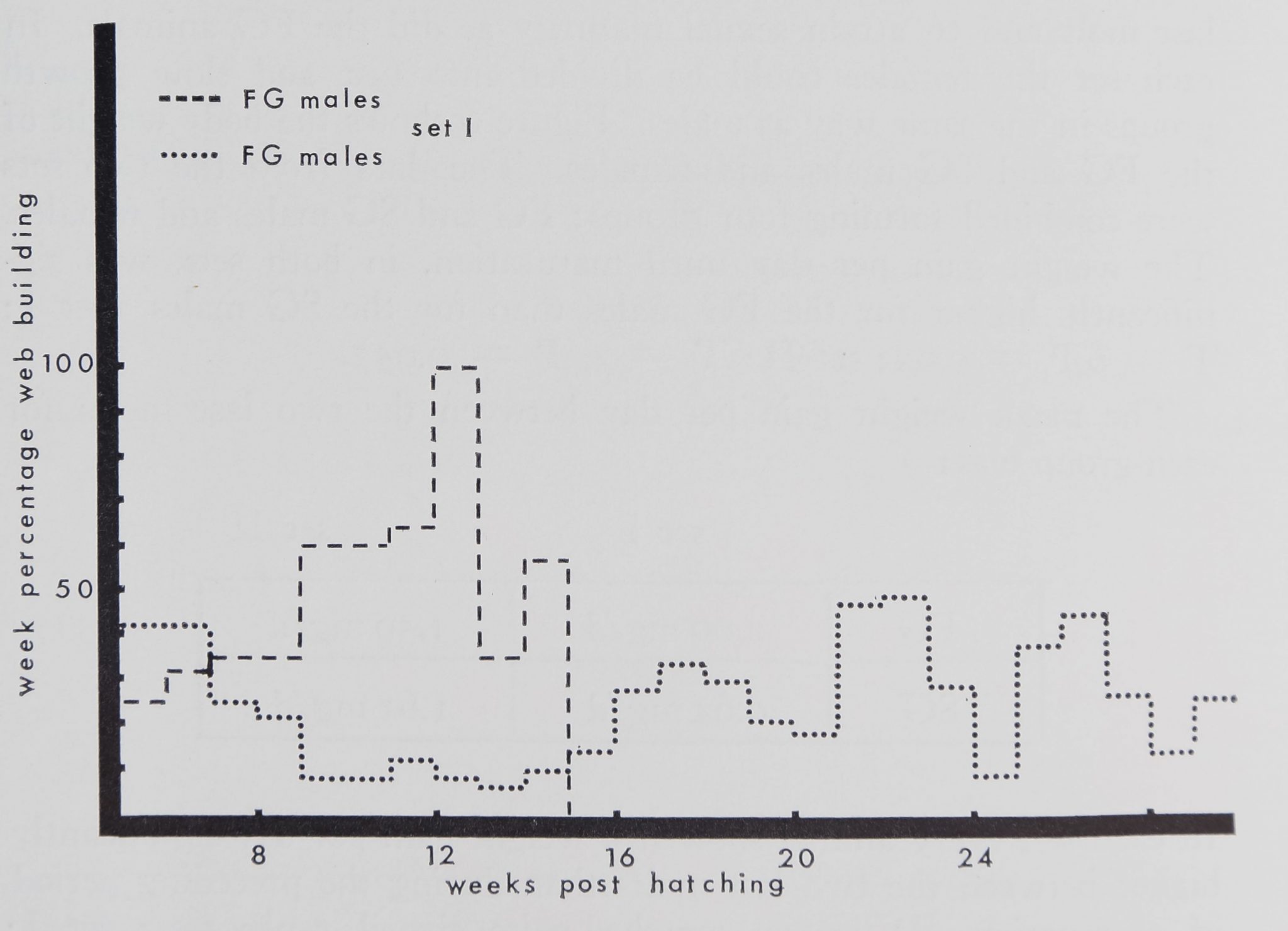
Figure 3. Frequency of building of the FG and SG males of set L
Dashed line: weekly mean of frequency of building for the four FG males.
Dotted line: weekly mean of frequency of building for the seven SG males*
Note the similarity in the pattern between weight increase and web building
frequency. (Compare with Fig. 2.)
Ramousse — A raneus diadematus
29
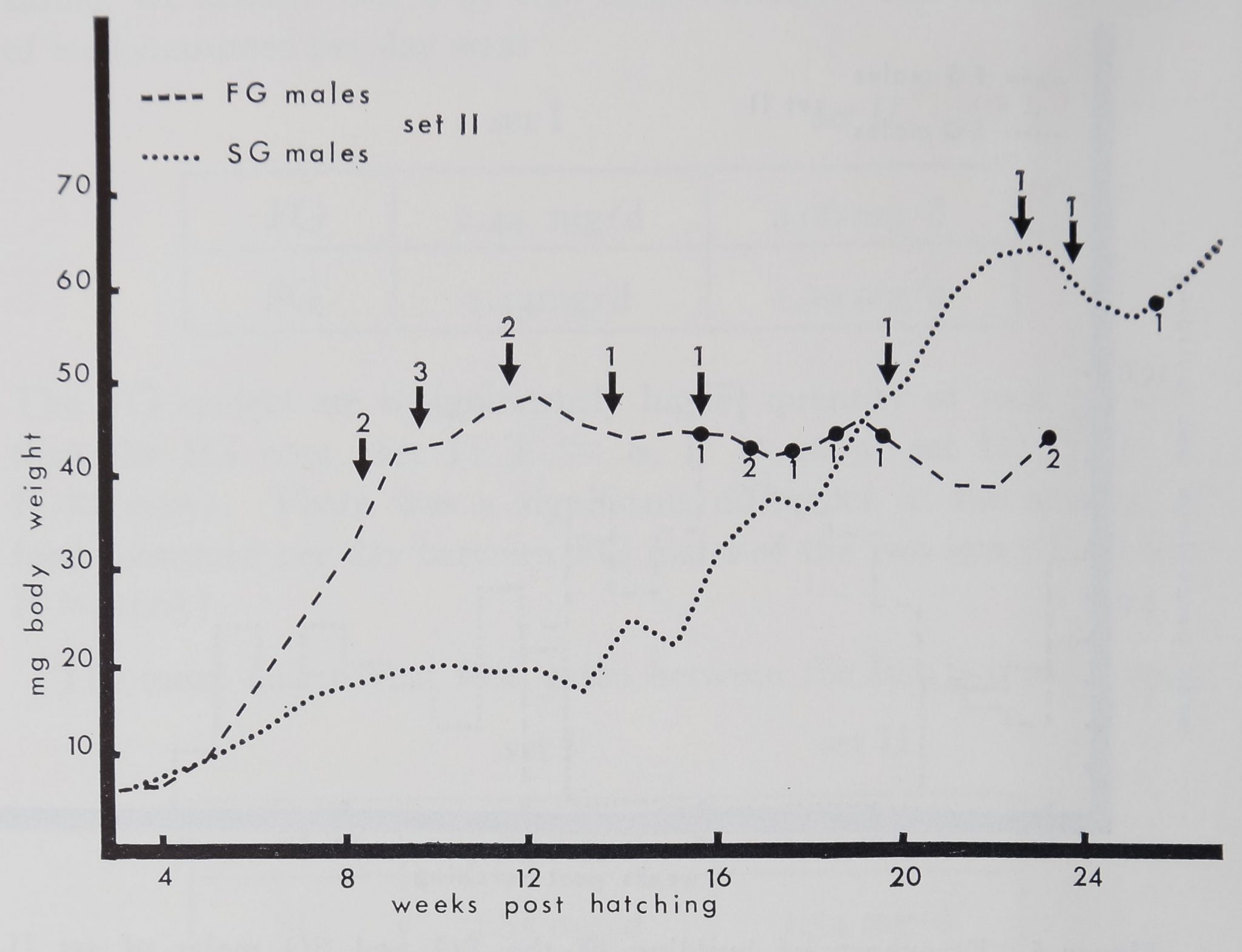
Figure 4. Body weight of 14 male Iittermates in set II of Araneus
diadematus hatched in the laboratory on March 6, 1972. Dashed line:
weekly mean body weight for the 11 FG males. Dotted line: weekly mean
body weight for the three SG males. Numerals followed by an arrow
indicate the number of animals molting for the last time during a week.
The FG animals reached their maximum weight the 12th week post-hatch-
ing, the SG males reached their maximum the 27th week post-hatching,
when the FG males are dead. (Compare with Fig. 2.)
The rate of building :
set 1 set II
FG 0.57 w/d 0.64 w/d
SG 0.31 w/d 0.30 w/d
between the two last molts was significantly higher than the rate of
building during the previous stages of growth in both sets (set I:
N = 9, T = 2, P = 0.01 ; set II : N = 12, T = 1.5, P = 0.01
Wilcoxon test).
What explanations are there for differences in frequency of build-
ing? A multiplicity of factors have been found to have some in-
fluences on web-building: a change from dark to light, a steep rise
Psyche
[March-June
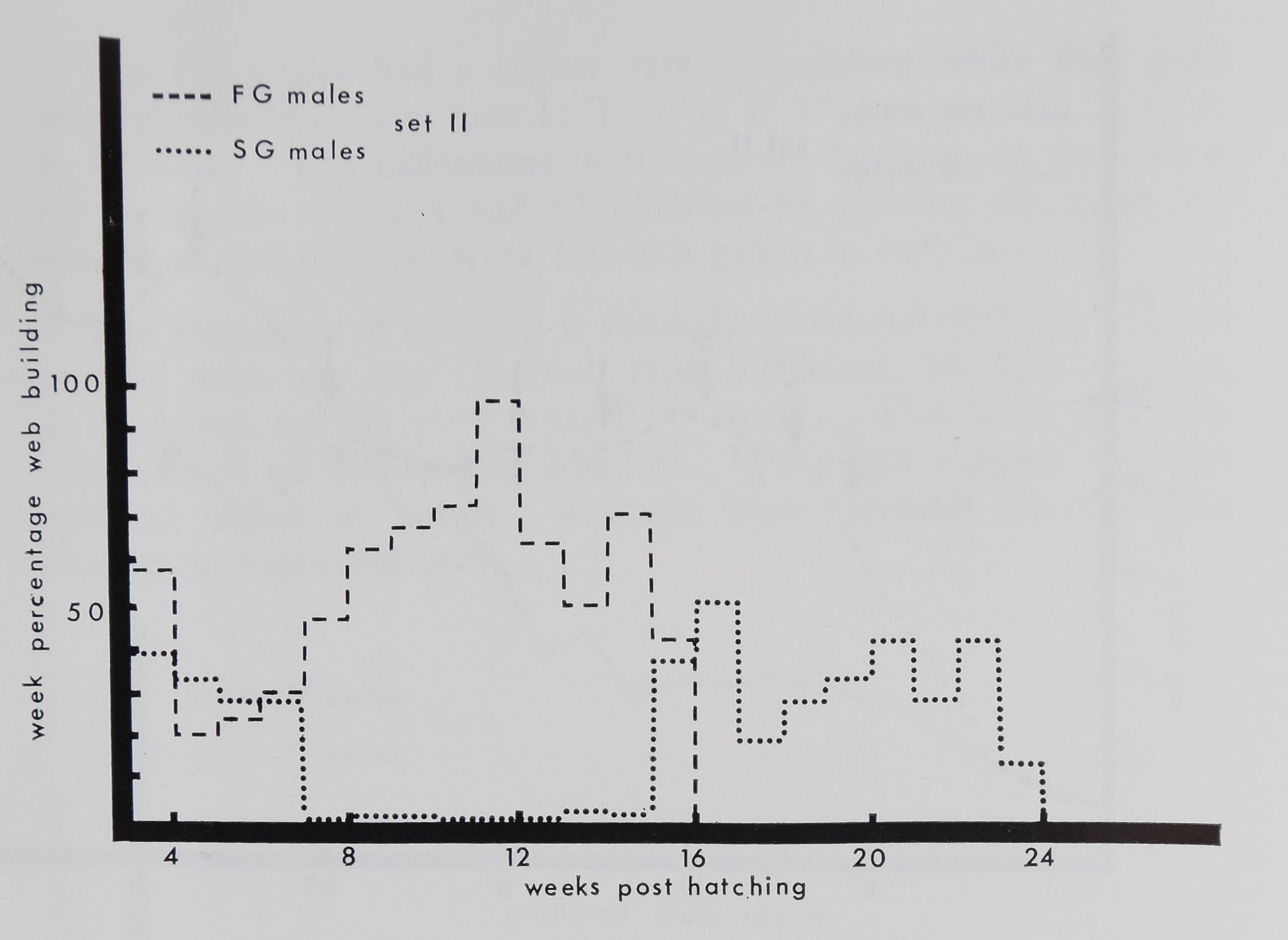
Figure 5. Frequency of building of the FG and SG males of set II.
Dashed line: weekly mean frequency of building for the 11 FG males.
Dotted line: weekly mean frequency of building for the three SG males.
Note similarity to Fig. 3.
in temperature following a temperature minimum, weather condi-
tions, barometric pressure, a full silk supply, hunger (Witt, et ,
1968). In the laboratory, all the spiders were subjected to the same
environmental conditions, therefore the differences in rate of building
should be due to an internal state, such as hunger. There is a gen-
eral agreement in the literature that hunger is a strong drive for
web-building. Heavy feeding is followed by several days without
web-building (Koenig, 1951; Wolf & Hempel, 1951; Wiehle, 1927;
Peters, 1932). The interpretation is that the hunger drive is too low
for releasers like temperature and light to operate. On the other
hand, spiders deprived of food built almost every day (Peters, 1939)
and built webs even at the expense of other body constituents (Witt,
1963b). We may assume that the FG males have a higher level of
hunger than the SG males, which induces a higher rate of building.
Food consumption
Each time a spider was fed, the fly was weighed before eating.
Since only one or two percent of a fly was rejected by a spider after
1973] Ramousse— Araneus diadematus 31
eating, we assume that a fly was eaten entirely. The mean quantity
of food consumed per day was :
set I set II
FG 2.44 mg/d 2.06 mg/d
SG i.44mg/d 1.39 mg/d
The FG spiders ate a significantly higher quantity of food per day
than the SG ones (set I: T = 6, P — 0.05; set II: T == 8,
P — 0.05 ). There was a significant difference in the amount of
food consumed per day between FG males of the two sets (T = 8.5,
P — 0.05).
The mean quantity of food eaten between the last two molts was:
set I set II
FG 3.33 mg/d 2.61 mg/d
SG 2.85 mg/d 3.54 mg/d
In each set the mean quantity of food consumed per day between the
last two molts was significantly higher than the mean amount of
food eaten per day during the preceding observation period, (Wil-
coxon test: set I : N = 10, T = o, P = 0.01 ; set II: N 13,
T = 1, P = 0.01).
A relationship exists between the amount of food eaten per day
and the growth rate in both sets, indicating that the growth rate is
a function of the amount of food consumed ( Kendall rank coefficient ;
set I: y = O.55, P = 0.01 ; set II: y = 0.60, P = O.OOi). The
foot eaten was used to sustain the basal metabolism, to make silk,
and to build the body of the spiders. A rough estimate of the per-
centage of food transformed into spider tissues was obtained by
dividing the gain of body-weight per day by the quantity of food
consumed per day: the FG males used about 57% (set I) and 47%
(set II) of the food they ate, while the SG males transformed only
33% (set I) or 32% (set II) of their food into spider tissues. The
FG groups transformed a greater amount of food consumed into
spider tissues than did the SG groups (set I: T = 6, P = 0.05;
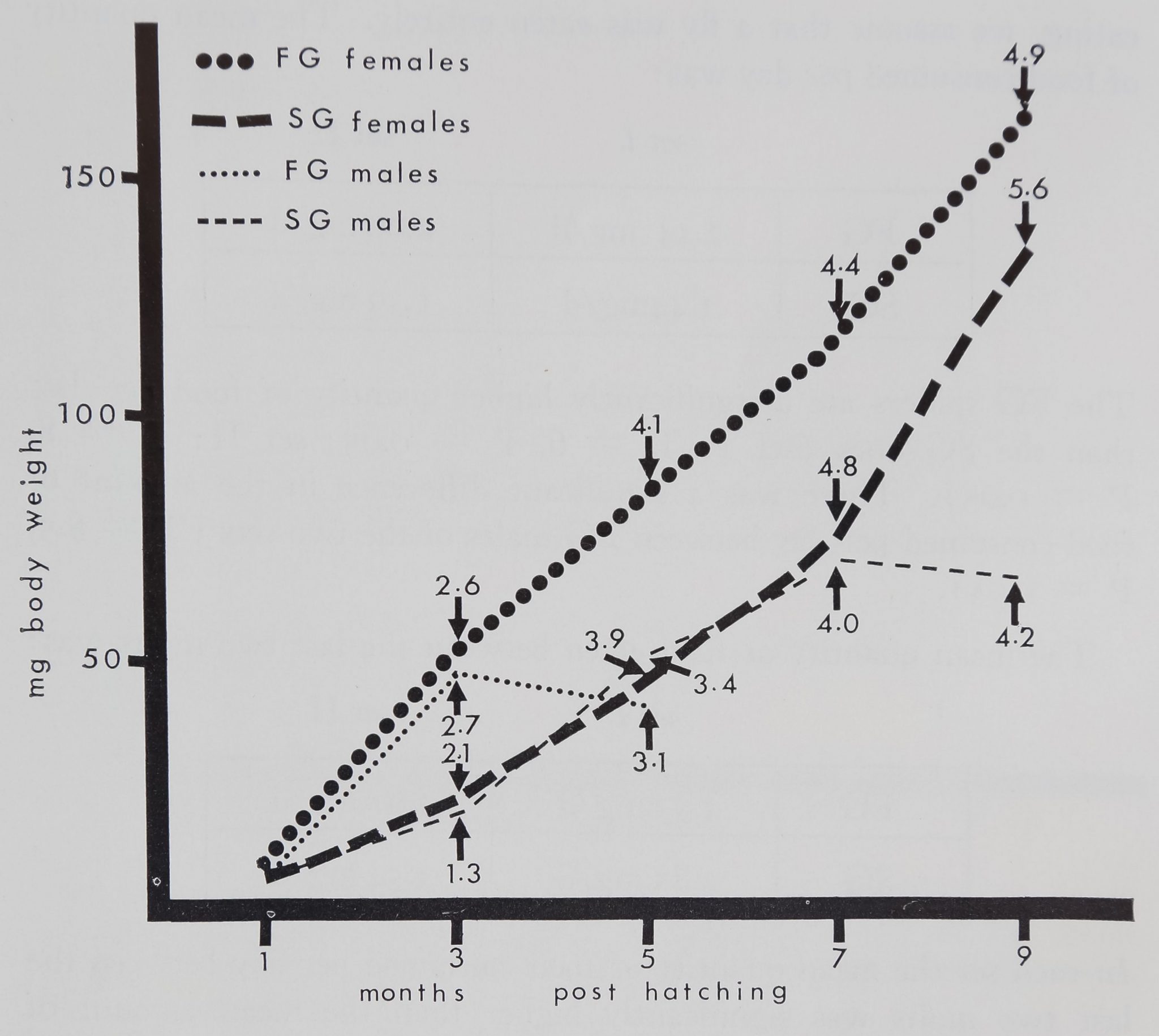
Figure 6. Body weight and number of molts of 25 males (15 FG, 10 SG),
and 25 females (15 FG, 10 SG) from the two sets cocoons of Araneus
diadematus studied. Each line connects mean body weights at one, two,
five, seven and nine months poist-hatching. Large black circles: FG females,
large dashed line: early life of SG females; small black circles: FG males,
small dashed line: SG males. Arrows indicate the number of molts to the
time. Note the different growth rates and the related1 different speed of
maturation in FG and SG males and females, and the similarities for
both sets.
set II: T = 14, P = 0.05). As a result of having more food
available for metabolism, an FG male was able to utilize more energy
for other metabolic processes than basal metabolism, such as synthesis
of silk, synthesis of body constituents, etc. This would assure a
larger supply of silk for the FG spiders than for the SG, which
could be an important drive for web-building (Peakall, 1967)* * he
increased frequency of building in the FG spiders leads to a greater
amount of food consumed which in time results in the rapid weight
gain and growth.
Ramousse — Araneus diadematus
33
1973]
Maturation
Between the start of the observations and the time of sexual ma-
turity (last molt) the mean number of molts recorded for each
group was:
set I set II
FG 3.25 molts 3.27 molts
SG 4.50 molts 3.66 molts
The SG males in set I went through a significantly higher number
of molts than did the FG males (T » 12, P ^0.05) and reached
a higher weight (see below). In set II, we had only three SG males
and one of them did not complete its development, this explains the
difficulty to obtain a significant difference between SG and FG ani-
mals in this set.
For set I the mean time of maturation was 81.6 days for the FG
spiders and 202.5 days for the SG spiders. In set II maturation was
reached in a mean time of 78.0 days for the FG males and 163.0
days for the SG ones. The time of maturation was significantly
longer for the SG animals (set I: T = 6, P — 0.05 ; set II:
T = 6, P.y? .0.01 ). In addition the time of maturation was sig-
nificantly longer for the SG in the first set than in the second set
(T = 6, P = 0.05).
The rate of maturation, number of molts divided by the number
of days necessary to complete these transformations, was significantly
higher for the FG males than for the SG males ( set I : T = 6,
P = 0.01 ; set II: T = 6, P = 0.05).
The Kendall rank coefficient between the gain of weight per day
and the number of molts per day was 0.61 for set I and 0.66 for
set II (in both P = 0.001). A relationship exists between the rate
of growth and the rate of maturation which is in agreement with
the findings of Deevey (194.9) with Latrodectus mac tans (Fabri-
cius) and of Benforado and Kistler (1973) with A raneus diadema-
tus. We may assume that the maturation rate is correlated with the
growth rate. The mean length of time in days between two con-
secutive molts was determined. In 3 out of 4 groups, the last inter-
molt was longer than the other intermolts ( table 1 ) ; for the FG
males, this last intermolt was significantly longer than the earlier
(N = 10, T = 1.5, P = 0.01 ).
Psyche
[March-June
Table i
I 2 3 4
set I FG 20.6 14.O
SG 63# lipf1;: 46.1 29.6
set II FG 22.7 ljg|jj|y —
SG Hi 1 1 17.0
lljean length of time lia- ‘Éàyf‘sepafa#Mg- two consecutive Molts. . The
numerals designate each intermolt’and its order in relation to the
final one, i being
increase in
The mean length, of the fjrst leg as measure^Æithe las| molt was :
PS I . M™ml
10.5 mm ‘ ;
SG */’ 14*3 fom 12.3 mm
SG m^srfv|^i we’re, legs
than |fG males after the j^|:,,ijiolt, ,(set I: T = io, P = o.oi ;
set II : T ‘^§§jg,
The rate of leg growth is givemhythe ratio of the lej^th fpin in
the number of necessary to obtain this incites, of length. The
mean rate of leg growth during the entire observation was.:.
set I . I
FG j. : 76 mm/d.. • y
SG O.O69 mUl/d d ‘ ■!
The rate of length increase was significantly higher for the FG
males than for the SG males (set I: I’SMi P ©.qi; set II:
T — 7, P — o,©5^ This points out the relationship existing be-
tween rate of maturation and rate of lengthening. No correlation
was found between the leg-growth between molts and the length of
time of the intermolt.
Maximum weight
Body weight increased for all males to a maximum at the last
molt, declining from this point onwards. This is in contrast to
Ramousse — Araneus diadematus
35
female weight increase which continues after the last molt, possibly
due to egg formation. The mean weight was :
set I set II
FG 62.95 mg 57.64 mg
SG 88.5^1i9^’4
The maximum weight reached by’the FG males, in both setgr was
lower than for the SG maJsfc? T’h^j suggests thffc the maximum
weight may be a ‘Jîjpctjoâ fjfjl the duration of devel©^®!^» In that
case, 1-apüd l&Kia^^HS^the’ WfeS^t growth.
No coirreiatiH between tpljmiî, weight and É^Bf||ight wasIgRprid
in contrast-^^^^pÿindings of ;®ehfcrâ^|i -.and Kistler A
relatively small dtf erenCe ^^nifial weights and the low accuracy-of
the wetgMàHÉ’-aMM.y’ the ‘êàÊÊÊmÊ^
Mating
The set post-hatehmg £jp-h.)
and ffinse.of set 11 ~ females- ~ reached ma-
turity 229 days p-h. in set I and 104 days p-h. in set II. In -the
first ftt: allj^Je FG “before any
were Mature, «^B’¥®nlftl|tg them. In the second ■«et, the
FG mateîi^Bè ï days after the fast that some of them
were still a|îf^when the females matured. But the females
accepted the àdyirsèes; M only ‘És> days or more, after the
last molQT^piN^enting the FG males from flgaifapg. wJii& their sisters.
In summary, mm FG |j|#b stefl-^ere seximMyi^M-atuc»© too
early to’fifate $ame,iÉÿ^:’f
The SG males reached iAturiQ^^®^^^^^K in’Set | and Ï63
days p-h. in set II. -A®- ifhf| time the FG females of. set II were
already mature (104 days ^Hijlp and those of set I were almost ma-
ture (229 days p-h.), as well ts the SG females of set II (223 p-h.).
In these conditions the SG male# of both sets may have been able to
mate with the FG females of’ their own set or the other set;
Each of the nine SG males, when they were an average of 300
days p-h., were brought into the presence of three different females.
All the males seemed to behave in the same way, but only three of
them mated successfully with a single female, and one with two
different females. These successful males were the biggest of the
SG males. One male of each set was able to mate with a female of
Psyche
[March-June
his own set. Only the FG females of the set I accepted the males,
while both FG and SG females of set II accepted the males. How-
ever, the small number of males limited the number of trials and
did not permit us to know, statistically which of the females (FG
or SG) Were the most successful fe gating. ;
The FG males cannot méw wfth females their own set, but we
may assume that they ‘dip. find females |#f d^ff^e^s in a natural
habitat witch arè ■ iêfïure at The* IG males can
mate FQ females ‘^^^^^^v^E^pêfmîflfeg~feiited in-
breeding. i Th%!|s«merêly< 8,^ different sets in nature
ïiiëy Save Vi/i|ii 1111’ ‘fn itiiilitihtf1
GèKÿptrfSWfi’ S G €$d. F,G Spiders
ihfe FG and SG ni-alts were compared on thf j^sis of
the- -sp^al/area,’ mesh-rlg^f. and ftf^ead length.
The spiral area of all webs hu.jhfchf^My|ef in;J|a||h groups and both
sets showêt. a general ifWrdaye u>w^ W»ing a ^^xinfiiîiÉ^fea during
the period between the^^M^Mm’ area decreased ih
^îgP ‘ dhereèftÿtt.:*- Ipifar males ^eftêi@jï®d. : earlier, whopMjd ritot
the? genéraj:web-buildinÿ P^P$ù#’^SB)||Wt “’web^’^Bl they diejdds
M0wsd ndifSirease in spiral $reaifn twpifinal Webs. ThHBurfher
supports s$^Ë*#ati0nthat they died before achieving full development
through a In ia^ip .thij. spiral area of
the ’ ‘females ê^’-\d<r€n,e.viS-_’• diadematusand Neoscona vertebrata in-
creased ih$|I|k;fhe. fast thrifei afte% wlji’fh Iftne .the1 e|jtèfiip€
•wm®- dkes’Kirot change & ,||aum, The
c&pàifajlf «HJ»’ of the females of the golden ^gafedCp-^^iégr,
aurantia,, shêfesi • a ^cprth ^nfelfel^nae, the ; peak jf<i#g. ;p^nciding
roughly time of and seiaual tt>®lgutftti<ao-i ( Reed, Witt
& Scarboro». ■tÿfâfyT.r^
s An essentially upward. Kneat ig^fyfh in mesh size throughout the
lifetime occurred £<xÊj^ of the males iStudied. For the 12 other
males the mesh size increased until reaching a plateau during the
last intermolt. Witt, Rawlings and Reed (1962) have painted out
that the mesh size of the female webs of diadematus show
also an increase until the last molt, and then reach a plateau. But
Argiope aurantia shows a linear growth in mesh size throughout the
lifetime (Reed, Witt & Scarboro, 1969)*
The thread length foKows the same pattern as the two other pa-
rameters with a peak during the last intermolt and then a decrease.
Argiope aurantia and Araneus diadematus females have been shown
Rammsse — A vcltlcus dicLdcincttiis
37
Figure 7. Web built by a young male of Araneus diadematus. Young
FG and SG males built webs with the same characteristics, small and fine-
meshed. The vertical white lines of the scale are spaced 20 mm apart.
Psyche
March-June
38
to change according to the same pattern for thread length (Reed,
Witt & Scarboro, 1969 ; Witt, Rawlings & Reed, 1972). The de-
crease of the thread length may be due to the thickening of the
threads as the weight ‘ip&ler increases (.Christiansen et al.,
1962). The males follow the same pattern of web-changes as the
females of the same species, Except for the catçhing area. The gen-
eral effect that young Amnens diadematus males build small,
find-meshed webs- ( Ftg. and d%#%^ the onset of the last inter-
molt bu-ilj| large,, wide-meshed wëbs; males at the end of the last
Mïtêrmol t;:-3|houf’ejfeagiriftg and leg-length significantly, build
mediofe – TbdifejHg^l appears ?À|.4. web size cannot
^^pl^pjOjgxplainedl*.”^© sip|;dgf.’sl bodily Witt, Rawl-
îrtgs & Res®|;, 19^ Reed,, Wift & Scatbo*, •;
0^^èaris&Mi>of the w$$%f of- the ~
Xft, the mme’ige
, All the ^<gte ^pojfc^raphe’d between the Jq$i and 1 weeks post
ha#£Mng FG rfiale^.f# both sets were measured. .-S9were the
w*MH§ eljprt’i^Gr mM|Ë^ ;|oth sets during ^ke same period of time.
All these spiders Were at the sf^^^ej, but thy^W%fFG spiders were
the before-reach-
ing maturation. the figures {foe spiral atf#; mesh ai^e,
thread fee regklar%y of tb^^p^htg of the
pEkeads (standard median1 mesh two
measures of the body, and of*.tpo web sig-
nificant diflffences between the -jM.’Jaitd SG males, w$||f the excep-
tion t^l^aïiance if the mesh ‘ the two groups
«how simfttr wgulariiijy, ■ ,
Table *
FG ” SG ■’t F
Body weight S0.1 ± tlJD mg 22.6 ± 6.4 mg 3.58 0.005
Leg length 9.1 ± 1.70 mm 6.68 ± 1.48 m 2.49 0.05
Spiral area 42,138 ± 9,883 mm2 13,956 ± 8,178 mm2 4.88 0.001
Mesh size S8.60 ± 6.96 mm2 34.75 ± 10.08 mm2 4.05 0.005
Thread length 16,066 ± 4,111 mm 6,950 ± 3,282 mm 4.07 0.005
SE median mesh size North 0.114 ± 0.030 0.162 ± 0.062 1.47
Measures of webs of the FG and SG males at the same age. Onlj t the
regularity measures in the last line are not significantly di fferent.
R an mus se—Araneus diadematus
39
Table 3
FG SG t p
Body weight 60.14 ± 11.56 mg 79.37 ± 18.07 mg 0.11
Leg size 10.75 ± 1.22 mm 13.6i|± 1.41 m 4.90 0.001
Spiral area 33,931 ± 10,131 mm1 08,766 ± 6,’559 mm8 Ul 0.400
Thread length 15,546 ± 2,768 mm 10,425 ± 1,715 mm 3 M’*’ 0.005
Mesh size 46.46 mm2 199 . 0*005
SE median mesh size 8J:$& – aooi
Measure of webs of the and SG males Ojtpm^arable stage of maturityi
It is neither possibles!# relate measures toi leg length
— the FG’s legs were^fSÿiftWl^ logger thablhe SG’s legs —nor
to maturation sinee at the last stages of matter^-‘
tion an<îvthé stages before. We may assume
that the gfrÉpbf the ^lèi’ ^ebï bu^Mir^heir regularity i^a function
of the r ll3|K4teS^ek Mm body dimensions.
4 ^ggiajtorMÂjLjwBjjfte JfàLf tÿSuBÿ
«pMt.tÂe same stage of matu
All the FG v^|V photographed during thgr last stage wer,e;»/fôm-
pared witlf aff w §G – webs photograpfred about : |«o days later,
during i||ir last stagetMl^%^gmpares webs btfHt ât different times
but comparable matuja^-v. Table 3V givfes the figures for
body weiaMTlfeg . aré% thread length, mesh sîze and the
variance of ’FMe mhs^^^.’i’Thh’ W%bs of the FG ( lighter-) ’ males had
a spiral area large®* a significantly longer thread, smaller mesh size,,
and a higher re^feti^ÿhan the webs Jfpilt l)ÿ -the SG males at
comparable and linger length’^ thread
produced by the FG piales than the SG males indicates that they
have a better suf$pMjj$f silk or thinner tbbe^fj^ This may be sup-
ported by the hi»ir ,amount of food. eate:)f”.,f|if’day and the higher
rate of utilization* of the food by the FG thakSG males. The larger
mesh size and irregularity of the is related to-f the larger
body dimensions of these ammals. The difference between the dimen-
sions of the bodies of the FG and SG males coincides with a longer
duration of development, for “‘the SG than, for the FG males. We
may assume that the regularity of spacing the spiral thread is related
to the duration of the development in the two groups of males with
different rate of growth, and is related to maturation within a group
having a homogenous growth rate.
Figure 8 (left). Web of an almost mature FG male, weighing 70.1 mg at this time.
Figure 9 (right). Web of an almost mature SG male, weighing 101.0 mg at this time. The web built by the
FG (lighter) male had a larger spiral area, a longer thread length, a smaller mesh size, and a higher regularity
than the web built by the SG (heavier) male. The two photographs are enlarged at the same scale, the vertical
white lines being originally spaced 20 mm apart. §
Psyche [March-J
Rammsse — A raneus diadematus
4i
Mortality
The mean mortality in each group was :
set I set. II
FG 161 .ô^daÿi* post-hatchttg ‘ ; J,*40f| days’ pdst-tmiching.
SG 3 dead (meaj| 1 post-hatching) §!#jtiïWlvipg( üliiiéadM print hpainKjl^ gteSdfedtepR post-hatdhing’ ■
than die JPp I:
T = 10, P = 0.01 ; set II fPQHfffeg.,I Rapid growth
occurs at the expense pf endurance which is in
findings that the spiders’ lives short-
ened _ food ^«aaifc^aiafc^^^^.littaKiiof. Reed and
Witt that the FG females of A raneus diade-
matus lived shorter than the SG females. ,
In%tfr labofâtôiyi the* ïrom Jîfly 1972
1973. B%t only
be’lÜÉiftfflîif1 in the Boston area,
fjMÜft ^Mfcûthètn Ai®^’ari4’«TOpnfi* (Bonttët
1935 ) • Nevertheless some authors found Araneus in the
field wetter ,t BertkaS<yi\S®1f) in
Germany •’^E Térkt^ÿftr talv. Millot ( igSiti-) also ‘.qft-
tained, in the laboratory, the survival of*y&(R JÎ
during wirttéf tffipy completed their develèpâfent :*;the. following
spring.
to thé environmental conditions .ft goes through, and a low jfattr ff|l
feeding statute1 aUdw~’à‘¥ông*er
lifespan fp? ‘ ihe spiders reared, in the lab^^lf^g’ than ip <j“hé, field.
In that case, the lengthening of th^ development merely emphasizes
the difference betwen FG and^S^an-irlii^te ®|
The FG males survived an average of ^jHafè1 after the last molt
in set I and 71.4 days in set II. These itiaieg. grew and built webs
approximately half their lives, then sought out mates. We can note
equivalent facts for the SG males with their relatively long time
scale.
Psyche
[March-June
General Discussion
The males grow un#, the last molt, at which time they attain
their maximum weight; weight decreases slowly thereafter. The
females have a distinctif different coiirse of growth; their weight
increased long after the last molt, generally until #ey Iftff a cocoon.
The males mature more rapidly than the females, but the females
grow bigger than the males and ‘live longer.
The females and the males of each of the two sets of Araneus
diadematus studied are clearly” divided into FG and SG. The FG
malesp%re characterized by a higher frequency of building, rate of
food ^^Mtyion, -càtg of weight increase,7 ratq-‘-#f leg growth, rate
of maturation as well as a smaller number’# molts than the SG
males, significant only forHt I in the last instance,
The positive correlation between tfie rate building and the rate
of^ffod consumptionj§|§?’ gp males must be expected, since the spiders
Wère ÿ0 only when’ they buft a web. The different frequency
if building>|pky”be explained as it lower threshold
through hunger in the FG than in the SG spiders. The number of
prey captured ja a function of behavior mechanisms of the spider and
potential prey; among the former are the stimuli that induce the
spider to attack, the efficiency of fhis- attack, and also a number, of
other variables such as} web-site, web-characteristics, and frequency of
building. TSf hunger stimulus- which induces both, r^e attack and
web-building : threshold for the FG than.ifpr the SG
spiders, suggesting that in a natural habi#|^the FG males would be
able fo capture.afd eat more food than the SG^mfeiris. ‘In addition,
the usual effect of genes on animals with rigid patterns is. to alter
behavior in a quantitati||| rather than a qualitative fashion (Mann-
ing, 1.967). The environmental cfoditions being the same for all
the animals, the difference in threshold of hunger may be the conse-
quence of different genotypes.
A relationship between the rate of food intake and the rate of
growth indicates that the food was converted into spider tissues, in
addition to maintain basal metabolism and to support the necessary
activities like prey catching. The percentage of food converted into
spider tissue was higher for the FG males than for the SG males,
explaining the different growth rates. The same mechanism could
provide a more ample supply of silk for the FG than for the SG
males, which is suggested by the analysis of the web dimensions of
Ramousse — Araneus diadematus
43
the two groups. Hunger is an important drive for web-building and
prey catching, which in turn increases the amount of food available
to the spider. As a consequence, a good supply of food permits the
spider to use more energy to metabolize tissues and silk, and a full
supply of silk lowers ‘ the threshold web-büilding. So, the fre-
quency of building may be controlled by a changed’ feed-back between
hunger and amount of fpod eaten.
A strong relationship exists between the, pg|g growth .and the
rate of maturation: But J* number of paolts fv$s not cons^^ nor
was the time Wççgs|ive molts. The FG males went
through fewer stages thap. the SG males, l^ignifîçàrat.;fnly in set I)
and in less time. The >%^dh|4>f the FG
spiders seems to to change more 6#S5heir rigid
skins. ” requi^^S^P^to overcomfc’ the
heavy*Wi?S6*T^)1 tilfe %Stta energy ift the
form-éf^elfef^eÿ î#or^ the The differential
mati attrHM^o^PPiBjNSn.’-^^^Pldtiïritioh l#a fûhé-
tion of fffife^noiunt of food eaten Pro”
fpçïfr^ in ^p^^^Éhipg, and SimilljQrela-
tions f«i«ytt t^telain the. différences ip development /between males apd
females m: well ,as. .between^ femal®m,|fhe same :3HT1§
Differetié’^âpSîfeles of* feeding Ifelhfe diftfeEëMiâf growth and
Benf^^fea.id Kistle-r,
1Q72) fiiïÿjrâMffîêiq’ that tl|^|i|^Mf food ehtoft lp^l de^^wnânt
fèu^^^Bué “with thé same amount of iood^vmMMtéjfhë ‘Spiders of
the same Iët show dififefeftt ffÿo%lrh%4téS’lln^ maturariort rât^ ^Reed
& Witt, 1972). This ^jjtejOaseiaiaidtiMy genetic conditions
controlyhe – development and maturation. In study, the. sn^eçs
could ;*$Mase»the food ‘-^«antitÿ’ • they need through behavior.
When thé ‘spSféfS’’ %rW|Miîentk^P|^^rohrdertim uclB^ibtis, ^ may
assume that the pMerëhc^M’bëhM^wpP^ffi^iip resent at hftehihg
time probably are genettéâlly1 dw^ftnmWd. One iftductes some
spiders (FG) to capture and paPftireTdod than other épidèrs, and
in turn this large amoun^^*food Paten by these âfdaèirs, increases
their rate of development’ and maturafïoh. The rapid growth in the
two sets occurs at the expense of endurance and maybe weight in-
crease, the FG males are short livers and small weighers. The short
life-span of the FG spiders prevent them from mating with females
of the same set, while some SG males live ‘long enough to mate with
the FG females of their own set.
Psyche
[March-June
Poetsdh has shown (1963), that cocoons of a single species of
spider hatch at different times. This presumably .provides an ad-
vantageous distribution of egg-pr<pdnction over a period of time. The
two cocoons studied hatched *.$1? dilferent ^irp#s^ ;and the males of
set II, which hatched fifteen, days grew fatter than the males
of set I (signi§çâ»t only, fpr Between the sets the
rapid gro»wlÉ8o.céurs«^fet:-at fiw e^%®e of, endurance and maybe
weight increase. Differentifl ‘ fu^between the istï as well
as within the f^Mbfîrfg tyfptrilyttpfjf of maftiM.^nimals over
a period1, rWf tMVdurfhg the favorable -seSSi
mature males’ and females ■can mate and- ;
Knfes. jwlfiPa bèttéj* uhance
^ The relative of males. favors -jyifeig between
^imaiy^^dffefont sets and of (ft^MB^ehavior instead of inbreed-
ing. This allows the soealeà, .to. consea^ a gen^je.>jpc^û^vMi high
jsefectivyàtentialMa^^^P^l^ip^M^B
^^Ibr^BffiiSdfeient. frotii the other
“stages. hdrHo#l«p.■ tta iIt. ÆM§. wejb§, ate^aiore food
peg^^R’rfhd grew- ‘ Jttè|ff; than * stages. T^e time
ie^r^^K^^g»tnwo rnMÉFflW ■gënérâliv-miÉIfer’ than the time
separating any>aa^^y^Éte^i?aaesiË^,r moite. Sexual
also took place during this per.fe^^^?.Wi|?p^^ssum.o,jge^i®t@@eEiiesis
too. Th^saBw^^BKcdkih, wdtjjpflie males niijjji food and a longer
thé-.•^l’.:j^^^Rte^topimi&nt. The.importance -of
the reqÿi’^mefifcs during this time must make it ,^e -. most difficult
-ffÈ, jhfjÈtfjff ■’■”
The -of diade^iatuf ,, hatched
at differenG®mfiplaced. int-qntlividual £p#nes> were studied in
the laboratory 4j*ring the ij^£$p[an-of thç males. During this timq>
the characteristics of the body..( weight -apd sizç,)^ the frequency and
the parameters of the webs, the number and date of the molts, and
the amount of food eatefi tyere recorded for each animal. The
spiders could choose their feeding schedules through their, building
behavior.
The males built and increased their weight only until the last
molt, in contrast to the females which continued both building
and increasing their weight long after the last molt. During the
Ramousse — Araneus diadematus
45
building period the males were distinctly different from the females
only during the last stage. The males lived shorter and grew less
than the females. The last intermolt was distinct from the other
stages: the males built more webs, ate more food, grew faster than
during the other stages.
Two different rates of development appeared among the males
of each set, determining a fast and slow growing group. The fre-
quency, the amount» f#od eaten, the rate of weight increase and
the rate of maturation higher for the fait growers than for the
slow growers. À§, a consequent offhe râfp^ growth, the life-span
of the fast growing males w a£’4hm^ ami lit maximum weight was
lower (|fu% not ‘ tKà’^^W%fhe slow growififs* males.
Hunger and amount the diffdr§imt growth
rates and, rel|tMd njâ#u€ition., rates ; % mphosed
for the |fet and
may be the consequence of a gefietic difference. Maturati^ would
be cofftrolid i^^lferemt’ -patèétÜll- behavior detetnS^KPtfti a
The differeMr^” set
and fee@rei|fa®^S, à distribution .off mature animatefover
gt yt&v. The rjdari^ quiets prevents yhe
fast ,grqyriag /naleg. from mating female,: $f the ^C^yrlefj’buff
limited, inbreeding impossible ..the w’ and
the females ofthe- same sg£. ( A potem-fiftt high of thf? species
is assured by the $f the dis-
persion dïff^réht
This ’W#rE^a#tBf>^Wftiît ,<jp tfiÿ. labo ^tories of the pivision of
Research, North Cathlifia PéparéSià-îf ifMwental Health , and was
supported by Granti’®ha|îïber Xf4frt«m> the National Science
Foundation to Dr. Peter Tl.dga:uthor gratefully ack-
nowledges the assistance of Dr.. Witt, during all stages, the assistance
of Mrs. Mabel Scarboro for all technical and laboratory work, Mrs.
Rubenia Daniels for her administrative assistance, and of Dr. John
O. Rawlings with whom the statistical tests used were discussed.
References cited
Benforado, J. and Kistler, K. H.
1973. Growth of the orb weaver, A raneus diadematus, and correlation
with web measurements. Psyche, 80: 90-100.
Psyche
[March-June
4t>
Bertkau, Ph.
1885. Ueber den Saisondimorphismus und einige andere Lebenser-
scheinungen bei Spinnen. Zool, Anz. 8: 459-464.
Bonnet, P.
1935. La longévité chez les Araignées. Bull. Soc. Etomol. de France.
40: 272-277.
Christiansen, A., Baum, R. and Witt, T. N.
1962. Changes in spider webs brought about by mescaline, psilocybin
and an increase in body weight. J. Pharmac. ex. Ther. 136:
31-37.
Deevey, G. B.
1949. The developmental history of Latrodectus mactans (Fabr.) at
different rates of feeding. Amer. Midi. Nat. Notre Dame, 42:
189-219.
Dobzhansky, T.
1951. Genetics and the origin of species. Columbia University Press.
Eberhard, W. G.
1971. The ecology of the web of Uloborus dwersus (Aranea: Ulobori-
dae). Oecologia (Berlin), 6: 328-342.
Enders, R.
1972. Web site selection by Argiope aurantia Lucas and other orb
weaving spiders (Araneidae). Thesis, N. C. State University,
Raleigh.
Koenig, M.
1951. Beitrâge zur Kenntnis des Netzbaus orbiteler Spinnen. Z. Tierp-
sychol. 8: 462-493.
LeGuelte, L.
1966. Structure de la Toile de Zygiella-x-notata Cl. (araignées, Agri-
opidae) et quelques facteurs qui régissent le comportement de
l’araignée pendant la construction de la toile. Thèse, Nancy.
Levi, H. W.
1971. The diadematus group of the orb-weaver genus Araneus north
of Mexico (Araneae: Araneidae), Bull. Mus. Comp. Zool.,
141(4) : 131-179.
Manning, A.
1967. Genes and the evolution of insect behavior. Jerry Hirsch-
McGraw-Hill (Behavior-genetic analysis).
Millot, J.
1926. Contribution à P histophysiologie des Aranéides. Bull, Biol. Fr.
et Belg,, Supp. 8: 1-238.
Peakall, D. B.
1964. Composition, function and glandular origin of the silk fibroions
of the spider Araneus diadematus Cl. J. Exp. Zool., 156 : 345-350.
1969. Silk synthesis, mechanism and location. Amer. Zoologist, 9: 71-79.
Peters, H. M.
1939. Über das Kreuzspinnennetz und seine Problème. Naturwissen-
schaften 47: 776-786.
PÔTZ8CH, J.
1963. Von der Brutfürsorge heimischer Spinnen. Wittenberg, Ziemsen.
Reed, C. F. and Witt, P. N.
1972. Growth rate and longevity in two species of orb-weavers. Bull.
Brit. Archnol. Soc. 2(6) : 111-112.
Ranvousse — Araneus diadematus
47
Reed, C. F., Witt, P. N. and Jones, R. L.
1965. The measuring function of the first legs of Araneus diadematus
Cl. Behavior 25: 98-119.
Reed, C. F., Witt, P. N. and Scarboro, M. B.
1969. The orb web during the life of Argiope aurantia (Lucas).
Devel. Psychobiology 2(2) : 120-129.
Reed, C. F., Witt, P. N., Scarboro, M. B. and Peakall, D. B.
1970. Experience and the orb- web. Devel. Psychobiology 3 (4): 251-265.
Sekiguchi, K.
1955. Differences in the spinning organs between male and female
spiders. Sci. Rep. Tokyo Kyoiku Daigaku, 8: 23-32.
Termeyer, R. M. de
1791. Richerche e sperimenti sulla seta dei Ragni e sulla loro gen-
erazioni. Scelte d’opusculi interessanti 3 : 288.
Turnbull, A. L.
1962. Quantitative studies of the food of Linyphia triangularis Cl.
(Aranea: Linyphiidae). Canad. Entomologist, 94(12): 1233-1249.
White, C.
1952. The use of ranks in test significance for comparing two treat-
ments. Biometrics, 8: 33-41.
Wiehle, J.
1927. Beitrâge zur Kenntnis des Radnetzbaues der Epeiriden, Tetrag-
nathiden and Uloboriden. Z. Morpholog. u. Okolog. der Tiere, 8 :
468-537.
Wolff, D. and Hempel, U.
1951. Versuche fiber die Beeinflussung des Netzbaues von Zilla-x-
notata durch Pervitin, Scopolamin and Strychnin. Z. vergl.
Physiol., 33 : 497-528.
Witt, P. N.
1963a. Interrelationships between web-building behavior and amount of
thread material in the spider Araneus diadematus Cl. Proceed,
of XVI Intern. Cong, of Zool.
1963b. Environment in relation to behavior of spiders. Arch of environ.
Hlth., 7: 4-12a-
Witt, P. N. and Reed, C. F.
1965. Spider web-building. Measurements of web geometry identifies
components in a complex invertebrate behavior pattern. Science,
149: 1190-1197.
Witt, P. N., Reed, C. F. and Peakall, D. B.
1968. A spider’s web. Problems in regulatory biology. Springer-Verlag,
New York.
Witt, P. N., Rawlings, J. O. and Reed, C. F.
1972. Ontogeny of web building behavior in two orb-weaving spiders.
Am. Zoologist 12: 445-454.