Images Collection
View this article in Search Friendly Plain Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
Reprinted from the Journal of Morphology
Vol, 141, No. 1, September 1973 © The Wistar Institute Press 1973
Histology of the Neurosecretory System and
Neurohaemal Organs of the Spider,
Argiope aur&ntia (Lucas)
K. SASIRA BABU 1
North Carolina Department of Mental Health, Division of Research,
Raleigh, North Carolina 27611
ABSTRACT Histological studies of the neurosecretory system during the post-
embryonic development of a spider, Argiope aurantia, were made at the light-
microscopic level.
Neurosecretory cells which are found in all stages are classified into type I
and type II cells. The type I cells are present in the aboral region of the brain
and in pedipalpal, ambulatory and abdominal ganglia of the subesophageal mass.
The type II cells which appear from the seventh stage are confined to the che-
liceral ganglia. Three stages of secretory activity (poor, medium and full) based
on stainability are described in these cells.
In both types clear axonal transportation of neurosecretory material is ob-
served. The discrete tracts and commissures formed by these neurosecretory
axons are described in the brain and subesophageal ganglion. The complexity
of some of these pathways is comparable to that of the ordinary neurons.
One pair of nerves from the brain and four pairs of nerves from the sub-
esophageal mass enter a neurohaemal organ, the Tropfenkomplex. This is a
paired structure, situated dorsally, on either side of the subesophageal mass.
The neurosecretory axons branch extensively within the organ and on their
course they form sacs or pools filled with secretory material.
The Tropfenkomplex is enveloped by a thin neural sheath which runs deep
into the organ dividing it into a series of lobes. Glial cells are distributed within
the organ. As in the neurosecretory cells, changes in stainability of secretory
material were also observed in the Tropfenkomplex.
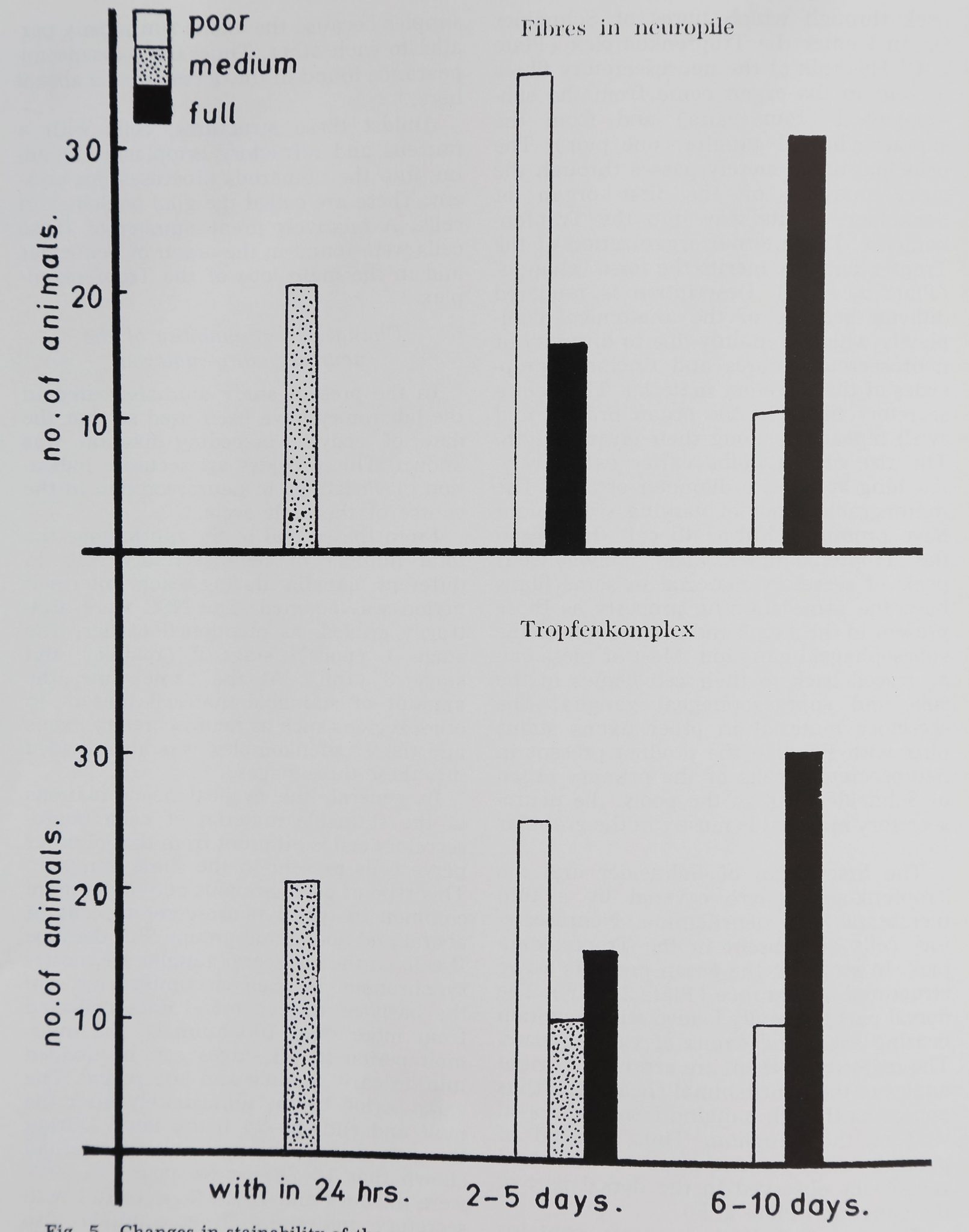
During intermolt periods two peaks of stainability have been noticed. The first
peak lasts for 24 hours after the molt, and this is followed by a low activity
period between second and fifth day. From the sixth to the tenth day after the
molt the second peak commences. It is suggested that the second peak may be
responsible for bringing about molting.
The cheliceral group appears ^seventh stage) at a time when external indi-
cation of reproductive characters are visible. In the ninth stage, by the tenth
day after the last molt, several of the type I and type II cells contain much se-
cretion. This is followed by maturation of gonads and oviposition. Thus both
type I and type II cells are believed to be involved in the reproduction of the
animal.
Our knowledge of neurosecretory sys-
tems in spiders is due to the work of Gabe
(’54, ’55); Legendre (’54, ’56a,b, ’59, ’64-
’66); Kiihne (’59); Streble (’66) and
Eckert (’67). These histological studies
were mostly descriptions of neurosecretory
cells in the brain and subesophageal gan-
glion. In the brain the neurosecretory path-
ways were incompletely described and
practically nothing is known about them
in the large subesophageal mass. Moreover
conflicting views were expressed regarding
the functional significance of the neuro-
secretory cells. It therefore seemed oppor-
tune to study in detail the cytomorphology
of the neurosecretory cells, the axonal
1 Present address: Department of Zoology, Sri Ven-
kateswara University, Tirupati, Andhra Pradesh, India.
J. Morph., 141: 77—98.
K. SASIRA BABU
pathways, the terminal depots for neuro-
secretory material and the probable func-
tions of the neurosecretory system in the
spider at various post natal stages.
MATERIALS AND METHODS
Female orbweb spiders, Argiope aurantia
Lucas (Family: Araneidae, see Levi, ’68)
were collected at Raleigh, N. C. The life
span of this spider ranges from eight to
eighteen months (Reed et al., *69) which
is advantageous for this kind of a study.
Cocoons were collected in the field in the
months of December and January. In
February arid March the spiderlings were
released into cages by cutting open the
cocoons. Each animal from the second
stage (“stage” refers to an intermolt
period; i.e., seventh stage after sixth molt)
was kept separately in a glass jar and data
sheets of the sex, age and time of molting
for each animal were maintained. The
younger ones, up to the fifth or sixth stage
were fed on gnats and the older ones on
houseflies. The laboratory provided a cycle
of short (eight hours) cool (18°C) nights
and long (16 hours) warm (24-28°C)
days throughout for the animals.
The first stage was brief ( approximately
two days) and the young ones underwent
their first molt within the cocoon. The total
number of molts for females varied from
seven to nine, the majority of them reach-
ing adulthood in five to six months which
is a normal period even for animals in the
field. No significant differences between
laboratory reared and field animals were
noticed. The majority of animals became
gravid and laid cocoons after the eighth
molt. Females died normally about four to
six weeks after laying the last of one to
three cocoons.
The entire céphalothorax was fixed in
Zenker’s, Holly’s or Bouin’s, after the
chelicerae, the legs and the abdomen had
been cut off. A total of 240 animals of all
age groups were used in this study. Twenty
to thirty animals were fixed for each stage.
During each intermolt period, animals were
fixed at regular intervals starting immedi-
ately after the molt to before the time of
the next molt.
Granular Histowax of M.P. 56-58°C was
used throughout. Sections of the entire
céphalothorax were cut at 8-10 p in the
three cardinal planes; very good paraffin
ribbons, even of the cuticle, were ob-
tained. Paraldehyde fuchsin-orange-G and
chrome haematoxylin-phloxine were exten-
sively used along with general stains like
Heidenhain’s haematoxylin, Azan and Mal-
lory’s phosphotungstic haematoxylin. Per-
formic acid/Alcian blue, Feulgen, PAS
with and without diastase and Gallocyanin
were used for histochemical tests. A
camera-lucida was used for making gross
sketches. The finer details of the axonal
pathways were filled into these sketches by
directly incorporating observations from
the slides. Making use of the horizontal,
sagittal and transverse serial sections,
neurosecretory pathways of the brain
and subesophageal ganglion were recon-
structed.
RESULTS
Neurosecretory cells
In the present investigation the serial
sections stained in paraldehyde fuchsin-
orange-G (PF) method gave very good re-
sults. Azan, Mallory’s phosphotungstic
haematoxylin methods also demonstrated
the occurrence of neurosecretory cells. But
the results with chrome haematoxylin were
poor.
Even though four types of secretory cells
were observed in the cephalothoracic nerve
mass, histochemical and other tests show
that only three of these are true nerve cells.
The fourth one may be a glial cell type.
Of the three secretory nerve cells, only two
were identified as true neurosecretory cells.
The third type may not be neurosecretory
in the strictest sense, and this will be ex-
plained later. The neurosecretory cell
possesses a nucleolus, prominent axon
hillock and an axon often filled with neuro-
secretory granules. On the basis of size,
cytomorphology and nature of the neuro-
secretory material, these cells are called
Type I and Type II. Legendre (’56a,b, ’59)
named these two groups Type A cells.
In the life span of the spider Argiope
aurantia relatively more groups of neuro-
secretory cells occur as the animals reach
subadult stages. In the present study, only
in the brief first stage were no cells ob-
served. In the second stage, the cells are
present in the aboral region of the brain
and in the four pairs of leg ganglia. In the
NEUROSECRETORY AND ENDOCRINE SYSTEMS IN A SPIDER
79
later stages, besides the above mentioned
regions, cells are also present in the gan-
glionic region (see fig. 1). Clear indica-
tion of the presence of these cells in the
pedipalpal ganglia becomes evident from
the fourth stage. In the cheliceral gan-
glionic region, the neurosecretory cells
make their apppearance after the sixth
molt or in the seventh stage. In general,
the occurrence of neurosecretory cells in
aboral, first leg, second leg, cheliceral and
abdominal ganglionic regions, is more con-
sistent and the stainability varies system-
atically not only in different age groups but
also during intermolt periods. In the
pedipalps, third leg and fourth leg, gan-
glionic regions, the stainability of the cells
varied greatly and hence no functional
significance could be attributed to their
activity. All the cells of the cheliceral gan-
glia, first and second leg ganglia and sev-
eral cells of the aboral and abdominal
ganglia, can be identified easily because of
their position, closeness to certain blood
vessels and bilateral distribution (see fig.
3). During peak periods of stainability, the
total number of active cells in different
ganglia of subadult and adult stages re-
mains nearly constant. The maximum
number of cells found in different ganglia
of sub adults and adults is: Aboral 16;
cheliceral six; pedipalpal six; first to fourth
leg eight; abdominal 25. An oral group of
neurosecretory cells was not observed.
Type I cells
The type I cells (Plate 1, A—D) are pres-
ent in the protocerebral and in all subeso-
phageal ganglia.
These cells show a continuous growth
from 2nd to the post cocoon stages. In the
second stage, the average cell diameter is
6 ^ with a nucleus of 4 In the post
cocoon stage, the cells increase to 12 ^
with a nucleus measuring 6.8 jx across.
Thus, the nuclear-cytoplasmic ratio of
0.66 in the second stage is reduced to 0.56
in the adults. The gradual increase of the
cytoplasmic area of the cells leads to a
corresponding increase of the amount of
neurosecretory substance synthesized in
the cell. Hence in the younger animals the
neurosecretory substance forms only a thin
ring around the nucleus. As the animal
grows older and as the cytoplasmic area of
the cell increases, there is a corresponding
increase in the amount of the neurosecre-
tory material. In adult animals, the neuro-
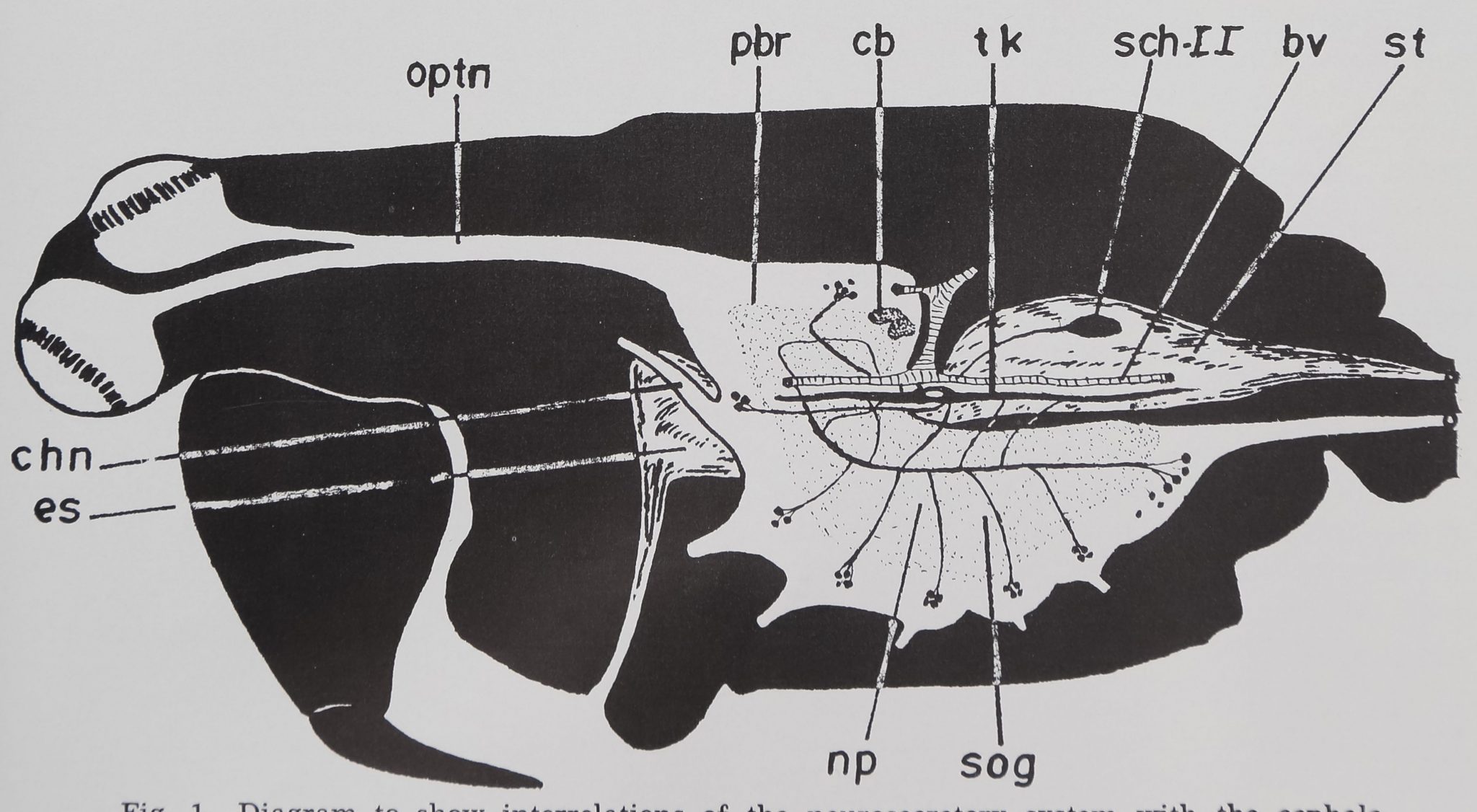
Fig. 1 Diagram to show interrelations of the neurosecretory system with the cephalo-
thoracic nerve mass and the retrocerebral endocrine organs in A. aurantia. bv, blood vessel;
cb, central body; chn, cheliceral nerve; es, esophagus; np, neuropile; optn, optic nerve;
pbr, protocerebrum; Sch II, Schneider organ II; st, stomach; sog, subesophageal ganglion;
tk, Tropfenkomplex.
80
K. SASIRA BABU
secretory substance fills up the entire cyto-
plasm of the cell.
The contour of the cell is greatly de-
pendent upon the quantity of the secretory
product present in the neurosecretory cell.
The stainability, as an indication of the
activity of the cells in different age groups,
can be divided into three important stages.
In the least active state (poor), the cell is
circular or oval and has a smooth outer
contour. The secretory material which
forms a ring around the nucleus is mostly
in the form of fine granules with a few
clumps here and there (Plate 1, A).
During this stage it is difficult to see any
secretory material beyond the axon hillock.
In the second stage of the cells, the amount
of granular material and the number of
clumps increase and fill up the entire cyto-
plasm, including^ the axon hillock. At
X 1000 magnification it is possible to ob-
serve the material beyond the axon hillock
of the cell. The neurosecretory cells develop
irregular outer contours which might be
due to the increased quantities of neuro-
secretory material within the cytoplasm
(Plate 1, B). During the maximal periods of
secretion (full), both the granular and
clumped secretory material fill the entire
cytoplasm (Plate 1, C, D). The irregular
contour of the cell increases. The secretory
material is prominently seen to the axon
hillock and also in the axons. During this
stage, within the proximal part of the
axon, the neurosecretory material is evi-
dent because of the small pools of the
secretory material formed at irregular in-
tervals, giving a beaded appearance to the
axon. In the fibrous central mass, along
with the smaller pools, here and there
larger pools of accumulated secretory ma-
terial were also found (Plate 2, A). As the
axons leave the ganglion more of the larger
pools are formed. These reach their maxi-
mum size and number in the Tropfen-
komplex.
Type II cells
The type II neurosecretory cells (Plate 1,
E) are larger and fewer (3 pairs) and con-
fined to the cheliceral ganglia. In the
seventh stage, at which time they begin
to secrete, the cells measure 16 /x with a
nucleus 8 ^ across. In the adult stage
these cells measure 30 ^ and possess a nu-
cleus 12 jx across. Thus, as in type I cells,
the nuclear-cytoplasmic ratio decreases, in
this case from 0.5 in the seventh stage to
0.4 in the adults. During the maximal
period of stainability, there is only a slight
change in the contour of the cell. The
secretory material stains lighter with PF
even during maximal production and is in
the form of small bodies distributed evenly
within the cytoplasm. When there is maxi-
mum secretion, vacuoles of different sizes
are readily observed in the perikaryon. The
secretory granules adhere or form a ring
on the margin of the vacuoles. The drop-
lets can be traced over long distances
within the prominent axons. Unlike in the
type I cells, even during low periods of
activity, neurosecretory material even in
axons is readily observable. Small granules
of the secretory substance can be seen
prominently close to the axon hillock. The
swellings or pools of secretion along the
course of the axons are almost absent. The
gradual increase of the axon diameter
distally, found in type I cells, is not
observed here.
The secretory product of both type I and
type II cells is acidophilic. Preliminary oxi-
dation of the sections with KMn04 gives
the product a very definite basophilia.
Histochemical tests show that this might
be a proteinaceous material since it stains
intensively with performic acid/alcian
blue. Sections treated with PAS gave nega-
tive results indicating that the product may
not be a polysaccharide. The neurosecreH
tory product also lacks affinity for gallo-
cyanin and therefore contains no ribonu-
cleic acids.
The third type of secretory nerve cells
mentioned earlier are the largest cells in
the central nervous system of the spider.
These occur in the cheliceral and subeso-
phageal ganglia. In adults, the cell diam-
eter varies from 30-50 ^ with a nucleus of
10-14 /x. There are three nucleoli, a promi-
nent axon hillock and an easily observable
axon that can be traced over long distances
into the neuropile and in several cases
right into the peripheral nerves. These are
the typical unipolar motor neurons found
in all invertebrates. The secretory material
in these cells appears from the seventh
stage and is present in increasing quanti-
ties as the animal grows older. These motor
NEUROSECRETORY AND ENDOCRINE SYSTEMS IN A SPIDER
81
neurons show densely stained areas of
rolled membranes within the perikaryon
(Plate lj F). The number of such lobed
areas varies from one to several, located
at different places in the cytoplasm. From
the lobed areas, granular secretory material
was seen released into the cytoplasm,
which reaches a maximum in gravid
females. In gravid females the secretory
material can be seen not only in the cyto-
plasm but also in the axon hillock and
axons.
These granules do not stain with per-
formic acid/alcian blue, but they give an
intensive PAS positive reaction. When
treated with diastase prior to the PAS reac-
tion, the stainable material disappears
completely. This indicates that the granu-
lar material stained with PF is most likely
glycogen. Such chemical compounds are
widely distributed in the animal cell and
thus cannot afford a histochemical defini-
tion of a neurosecretory product (Gabe,
’66).
The fourth type are clearly glial cells as
pointed out by Kiihne (’59 ). Legendre
(’56a,b) claimed these to be neurosecre-
tory and called them type B cells. In the
present investigation it was found that
these cells have no clear nulceoli, that the
nucleus is smaller than in nerve cells, and
that there are no axons. A number of pro-
cesses radiate from the periphery of the
cells, which occur at the edge of the neuro-
pile where glial cells are found normally.
Neurosecretory pathways
The characteristics of neurosecretory
neurons and their staining affinities to PF
made it possible to trace the neurosecretory
pathways. The axons which are loaded
with secretion, characteristically stain
darker with PF. This has made possible
the tracing of the course and extent of
the neurosecretory axons. The majority of
these axons travel in bundles both in the
brain and subesophageal ganglion, and
this makes the task of tracing much easier.
During the peak period of stainability, the
swellings and pools of secretory material
found along the course of neurosecretory
axons were of additional help. The neuro-
secretory tracts described below were re-
constructed from serial sections cut in the
three planes. The results were pooled by
observing serial sections from second to
post cocoon stages.
The paths followed by the neurons are
diverse. There are neurosecretory axons
that have a simple and straight course
from the soma to the neurohaemal organ.
But there are others whose pathways are
highly complex. These run through several
layers of neuropile and ganglionic regions
ipsilaterally or contralaterally and even
descend or ascend, before they finally
terminate in the neurohaemal organ.
Protocerebral neurosecretory pathways
The neurosecretory paths in the proto-
cerebrum present a picture of confusion.
This is primarily because of the single
fibres which run at random within the
neuropile. Hence no attempts were made
to follow the course of these individual
fibres. However, there are some well de-
fined tracts and the following description
applies to them.
The descriptions of protocerebral neu-
rosecretory tracts by Gabe (’55), Legendre
(’59) and Streble (’66) were incomplete.
Hence a detailed account of these tracts
is presented below. Neurosecretory axons
from each half of the aboral group of cells,
form a major tract called the protocerebral
neurosecretory tract (figs. 2, 3, pnt). The
tract from its dorsal position moves an-
teriorly and downwards into the neuropile.
In the middle region of the protocerebrum
there is crossing over of several fibres be-
tween the left and right tracts. After
decussation, which is here recorded for the
first time, the protocerebral neurosecretory
tract moves further downwards in an
aboral direction. This entire tract with
six to ten secretion-filled axons can be seen
clearly. Under high power and oil immer-
sion, the axons show swellings and occa-
sionally small pools of secretory material.
On either side of the esophagus, the pro-
tocerebral neurosecretory tract joins with
two subesophageal and one pedipalpal
neurosecretory tract. These tracts together
(10-25 fibres) move up along the posterior
margin of the brain to emerge out at the
junctional region of the trito- and proto-
cerebrum, as the principal nerve (figs.
2-4, pn) which enters the Tropfenkomplex
near the first organ of Schneider.
Another major neurosecretory tract in
K. SASIRA BABU
the brain whose origin is mainly from
neurosecretory cells of the subesophageal
ganglion is the subesophageo-protocerebral
tract (figs. 2, 3; spt). The subesophageal
neurosecretory tract in the pedipalpal
ganglionic region below the esophagus
divides into the subesophageo-protocerebral
and esophageal tracts. The subesophageo-
protocerebral tract from its central position
moves orally and dorsally up into the pro-
tocerebral region (fig. 3; spt). In front of
the central body, the tract moves down-
wards and joins the protocerebral neuro-
secretory tract as the posterior region of
the esophagus.
Cheliceral neurosecretory tracts
As described by Gabe C55), Legendre
(’59) and Streble (’66) the neurosecretory
cells in the tritocerebrum form a single
tract on either side of the esophagus. Each
bundle of fibres runs aborally along the
esophagus and joins the pharyngeal nerve
posteriorly. The nerve along with the secre-
tory fibers ends in the II organ of Schneider
(figs. 1-3). The secretory product in these
fibres is granular and the beaded appear-
ance or pools were not found here.
Neurosecretory tracts in the
subesophageal ganglion
The earlier workers (Gabe, ’55; Legendre,
’59; Kiihne, ’59; Streble, *66) were con-
tent with description and distribution of
the neurosecretory cells in the subeso-
phageal ganglion. Until now, the organiza-
tion of neurosecretory pathways and their
eventual termination in a neurohaemal
organ have been unknown. A nearly com-
plete description of the organization of the
neurosecretory pathways is presented
below.
The neurosecretory axons from each of
the ganglionic regions form discrete
bundles and well-organized tracts and
commissures that can be followed in PF-
stained serial sections (fig. 1). In each half
of the subesophageal ganglion the single
largest tract formed by neurosecretory
fibres from different ganglia is the longi-
tudinal subesophageal neurosecretory tract
(figs. 2, 3; snt). The position of this tract
corresponds to the central tract of Poecilo-
theria (Babu, ’65). The subesophageal neu-
rosecretory tract increases in size gradu-
ally from posterior towards the anterior
end of the ganglion. In the pedipalpal
region, close to the esophagus it bifurcates
into the subesophageo-protocerebral and
the esophageal tract (figs. 2, 3; spt, ot).
The path of the subesophageo-protocere-
bral tract was described earlier. The eso-
phageal tract, on the other hand, runs
aborally along with the esophagus within
the pedipalpal ganglia and finally joins the
principal nerve on the same side.
The subesophageal neurosecretory tract
is formed from axons contributed by neu-
rosecretory cells in the abdominal, ambula-
tory (4 pairs) and pedipalpal ganglionic
regions. Fibres enter the tract ipsilaterally
and even contralaterally from all the
ganglia. The majority of the axons are of
ascending rather than descending type.
Neurosecretory tracts of the
first leg ganglion
The distinctive cell groups in the pedi-
palps, legs and abdominal ganglia form
identical tracts and commissures before
joining the subesophageal neurosecretory
tract or the neurohaemal organ (fig. 2).
Since these tracts and commissures are
more pronounced in the first and second
leg ganglionic region, the present descrip-
tion applies mostly to one of these regions.
The three to four compactly placed neuro-
secretory cells in each half of the ganglion
can be identified easily because of their
distinctive staining reactions. It is normally
difficult to find the proximal parts of these
axons, but during maximal periods of
secretion the beaded appearance makes it
possible to trace the axons from the cell
body right into the neuropile. The axons
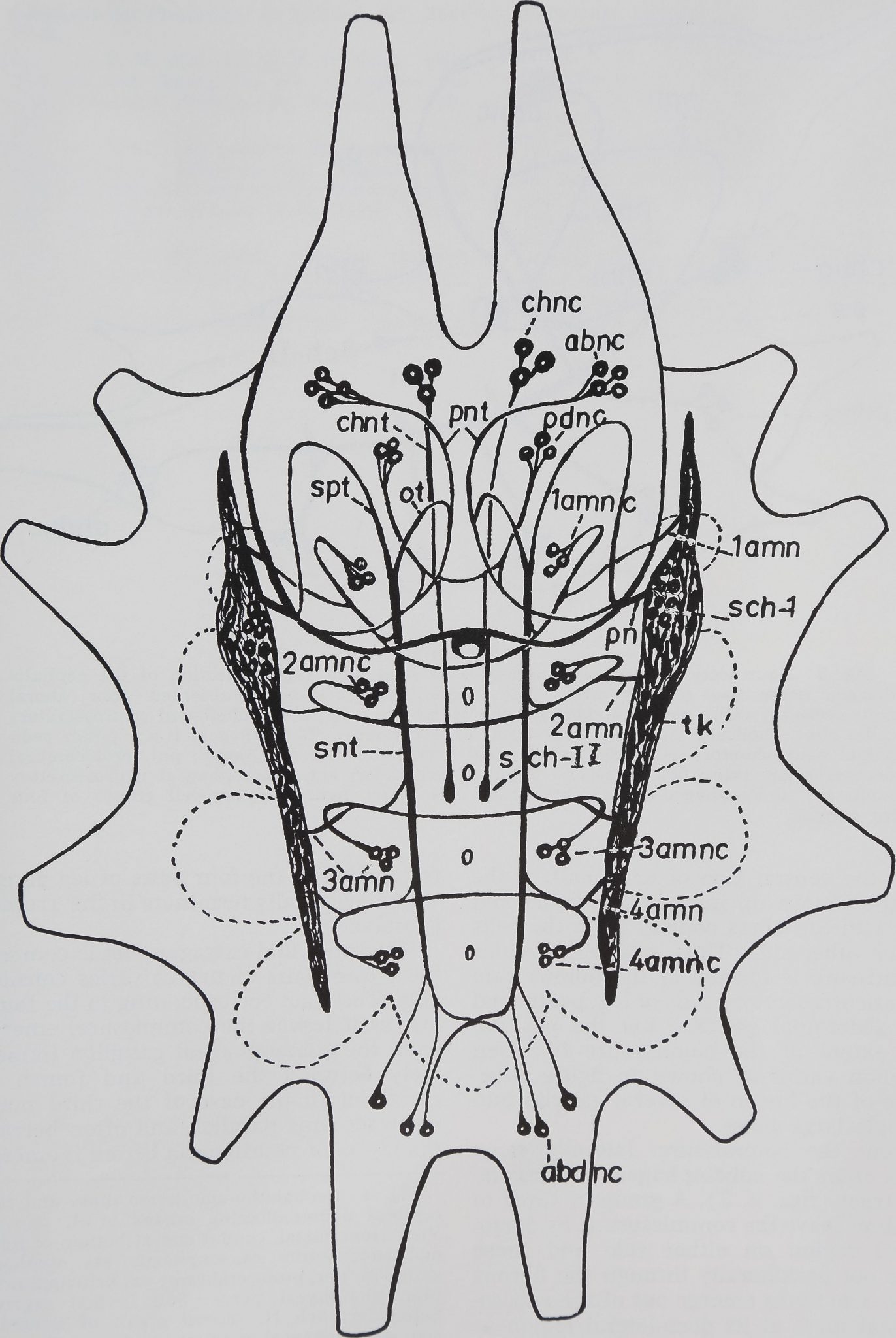
Fig. 2 Neurosecretory cells and pathways in
the brain and subesophageal ganglion of A. au-
rantia. The neurohaemal organs, with their in-
terconnections, are also shown in the diagram.
abnc,i;| aboral neurosecretory cells; abdnc, ab-
dominal neurosecretory cells; chnc, cheliceral
neurosecretory cells; chnt, cheliceral neurosecre-
tory tract; ot, esophageal tract; pdnc, pedipalpal
neurosecretory cells; pn, principal nerve; pnt,
protocerebral neurosecretory tract; Sch. I; first
organ of Schneider, Sch. II, second organ of
Schneider; snt, subesophageal neurosecretory
tract; spt, subesophageo-protocerebral tract; tk,
Tropfenkomplex; 4amc, neurosecretory commis-
sure of the fourth leg ganglion; 1-4 amn, am-
bulatory neurosecretory nerves corresponding to
four leg ganglia; 1—4 amnc, neurosecretory cell
groups corresponding to four leg ganglia.
NEUROSECRETORY AND ENDOCRINE SYSTEMS IN A SPIDER
83
Figure 2
84
K. SASIRA BABU
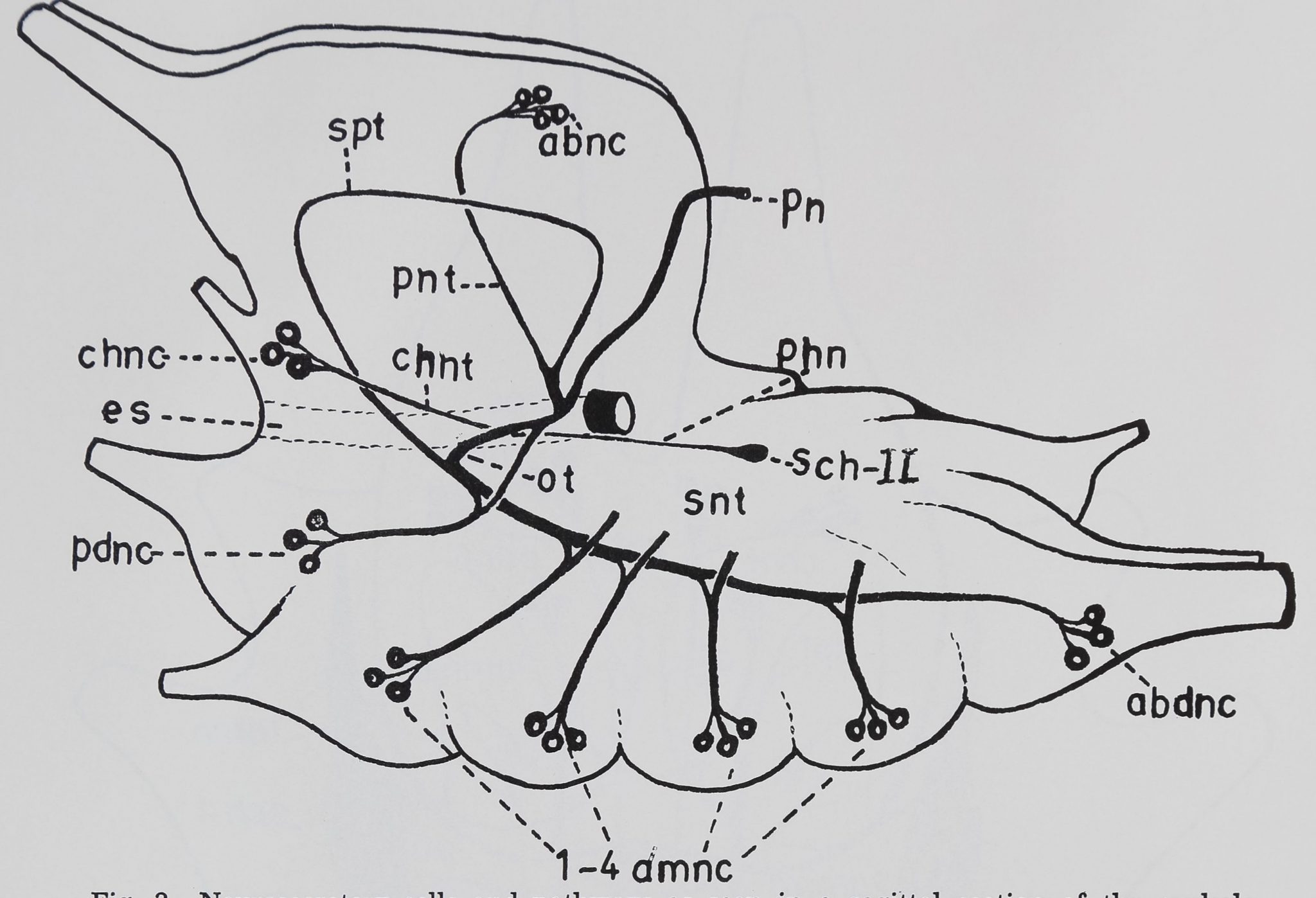
thoracic nerve mass of A. aurantia. The Tropfenkomplex is not represented, abnc, aboral
neurosecretory cells; abdnc, abdominal neurosecretory cells; chnc, cheliceral neurosecretory
cells; chnt, cheliceral neurosecretory tract; es, esophagus; ot, esophageal tract; pdnc, pedi-
palpal neurosecretory cells; phn, pharyngeal nerve; pn, principal nerve; pnt, protocerebral
neurosecretory tract; Sch II, second organ of Schneider; snt, subesophageal neurosecretory
tract; spt, subesophageo-protocerebral tract; 1—4 amnc, neurosecretory cell groups of four
leg ganglia.
from the ventral side of each half of the
ganglion move up into the neuropile and
join with the fibres coming from the cells
on the other side. Thus, one semicircular
commissure is formed in the fibrous core
from neurosecretory cells of leg, pedipalpal
and abdominal ganglia. But the position
and extent of the commissure for each
ganglion varies as shown in figure 2 be-
cause of the fusion of several ganglia into
a single large mass.
From the commissure, laterally some
fibers enter the subesophageal neurosecre-
tory tract (figs. 2, 3). A group of three to
six fibres leave the commissure at its dorso-
lateral region on either side and these
move out peripherally through the fibrous
mass. The fibres emerge out of the subeso-
phageal mass at its dorsolateral region as
ambulatory nerves (figs. 2, 3; 1-4 amn).
Thus there are four pairs of nerves cor-
responding to the four pairs of leg ganglia
which eventually terminate in the Tropfen-
komplex.
The intra- and extraganglionic course of
these four pairs of nerves varies consider-
ably. The tract corresponding to the fourth
leg, as it leaves the commissure, emerges
from the subesophageal ganglion immedi-
ately between the third and fourth leg
ganglion. In the case of the third nerve,
the tract runs parallel, and often between
the layers of neurilemma before it emerges
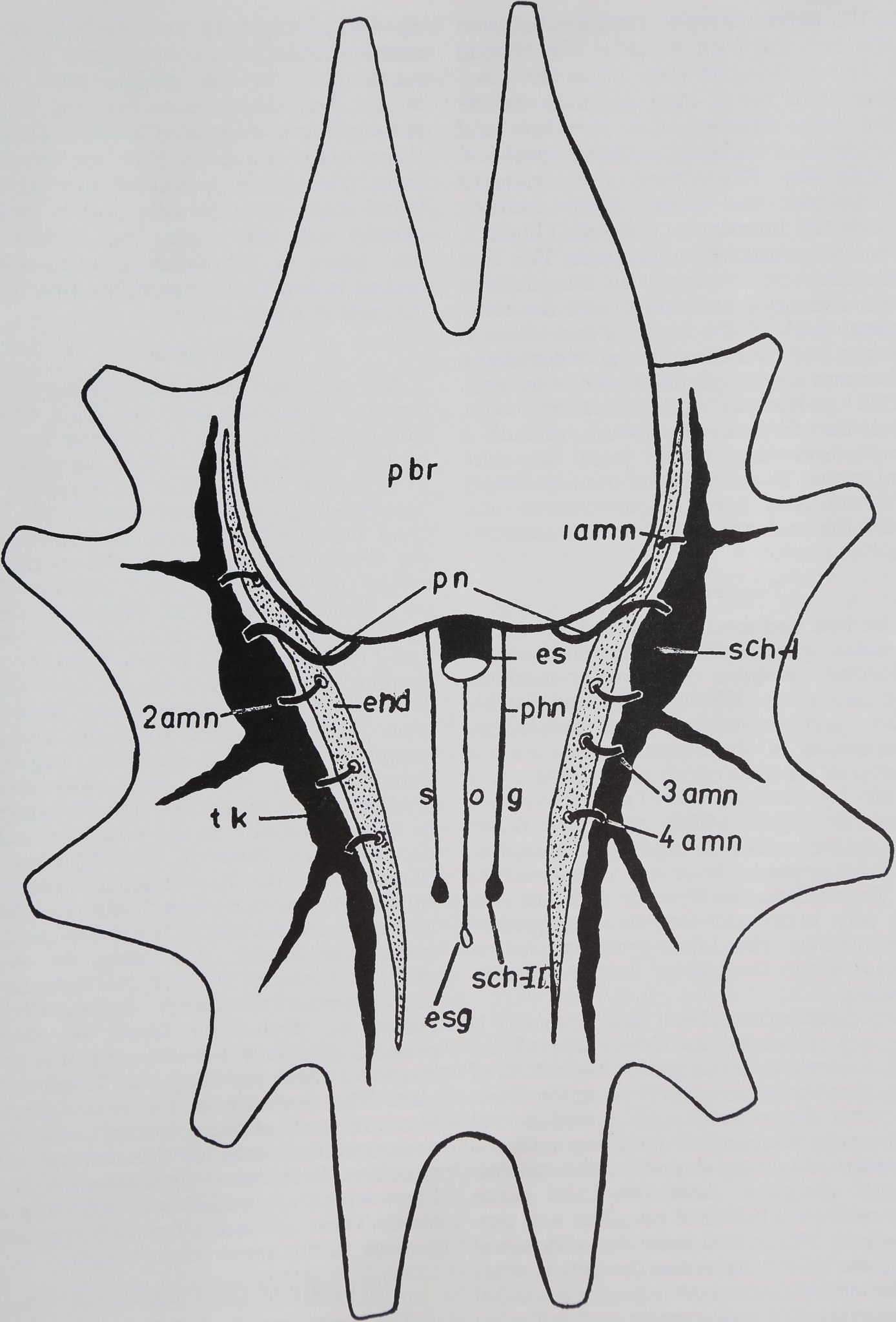
Fig. 4 Cephalothoracic nerve mass and retro-
cerebral neuroendocrine organs of A. aurantia.
View from dorsal, caudal end at bottom of figure,
end, endosternite; es, esophagus; esg, esophageal
ganglion; pbr, protocerebrum; pn, principal nerve;
phn, pharyngeal nerve; Sch I, first organ of
Schneider; Sch II, second organ of Schneider;
sog, subesophageal ganglion; tk, Tropfenkomplex;
1-4 amn, ambulatory neurosecretory nerves cor-
responding to four leg ganglia.
NEUROSECRETORY AND ENDOCRINE SYSTEMS IN A SPIDER
85
Figure 4
86
K. SASIRA BABU
from the subesophageal ganglion between
second and third leg ganglia. The second
and first nerves, as they leave the com-
missure rim for a long distance in the
fibrous mass showing large swellings and
great pools of colloidal secretory material
on their way. The second nerve emerges
just behind the brain after running
through the neurilemmal layers (Plate 2,
B) for a considerable distance. The first
nerve also runs through the neurilemma
of the subesophageal mass and the trito-
cerebral part of the brain. From there it
emerges and cuts through the endosternite
and enters the Tropfenkomplex anteriorly.
The pedipalpal neurosecretory cells,
which also form a commissure, give off a
dorso-lateral tract which joins the prin-
cipal nerve. The abdominal neurosecretory
cells, similarly form a commissure and
enter the subesophageal neurosecretory
tract.
Tropfenkomplex
The first and second organs of Schneider
constitute part of the retrocerebral neuro-
endocrine complex. The earlier workers,
Legendre (’59), Kühne (’59) and Streble
(’66), gave anatomical and histological
descriptions of these two organs. An ex-
tension of the first organ of Schneider was
called by Kühne the Tropfenkomplex.
Schneider (1887/1892) who first found
this organ called it marginal ganglion.
But it is appropriate to call this structure
the Tropfenkomplex since it does not con-
tain any nerve cell bodies. The present
study shows that the principal neuro-
haemal organ in Argiope is the Tropfen-
komplex.
The Tropfenkomplex (figs. 2, 4; tk) is
a paired structure, on either side of the
cephalothoracic nerve mass. Each half of
the organ lies in an anterio-posterior direc-
tion with the anterior end placed lateral
to the supraesophageal ganglion and the
posterior end lying dorsal to the subeso-
phageal ganglion. Anteriorly, the organ
ends near the cheliceral ganglion and pos-
teriorly it terminates near the abdominal
ganglion. Along its entire length, it rests
on the endosternite and is partly encircled
by extensions of endosternite and radiating
muscles. The diameter of the organ varies
considerably in the middle region (from
40—100 //,) tapering towards both ends. A
series of transverse constrictions are found
particularly in the middle part of the
organ. The sheath runs inwards dividing
the organ into a series of lobules. There are
three main branches that are given off
from the middle region of the organ. A
blood sinus runs dorsally and is in close
contact with the organ (fig. 1; bv). The
first organ of Schneider is intimately at-
tached to the Tropfenkomplex just behind
the protocerebral region.
Innervation
The accessory nerve innervating the first
organ of Schneider from the brain and the
interganglionary nerves between first and
second organs of Schneider, as described
by Legendre (’54), could not be found. The
Tropfenkomplex is innervated by the prin-
cipal nerve which leaves the brain near
the tritocerebral region as was mentioned
earlier by Legendre C54), Kühne (’59)
and Streble (’66). The Tropfenkomplex,
as the present study shows, is also inner-
vated by four pairs of nerves from the sub-
esophageal ganglion (fig. 4; 1-4 amn).
The first pair of nerves whose path has
been described earlier, joins the Tropfen-
komplex anterior to the first organ of
Schneider (fig. 4; 1 amn). The second
pair of nerves enters the Tropfenkomplex
in close proximity to the posterior end of
the Schneider Organ I. The third pair of
nerves joins the neurohaemal organ be-
hind the Schneider Organ I (fig. 4; 2 amn)
and near the middle of the subesophageal
mass. The fourth pair enters the organ
near its posterior bifurcation, anterior to
the abdominal ganglionic region. All four
nerves on their way from the subeso-
phageal ganglion cut through the endo-
stemite before reaching the Tropfenkom-
plex. The majority of the neurosecretory
fibres in each of these nerves come from
neurosecretory cells of the corresponding
ganglion. Each nerve contains three to six
fibres which are loaded with secretory ma-
terial. There are also a few ordinary axons
present in the same nerve bundle.
Structure of the Tropfenkomplex
The first organ of Schneider and the
Tropfenkomplex even though inimately
interconnected, are separated by a small
NEUROSECRETORY AND ENDOCRINE SYSTEMS IN A SPIDER
87
neck through which fibres of Schneider
Organ I enter the Tropfenkomplex (Plate
2, C. The bulk of the neurosecretory fibres
present in the organ come from the sub-
esophageal (four pairs) and from the
supraesophageal ganglia (one pair). The
principal nerve merely passes through the
outer margins of the first organ of
Schneider, on its way into the Tropfen-
komplex. The internal organization of the
Tropfenkomplex merits the term “chaotic”
(Plate 2, E, F). Description is rendered
difficult because of the anatomical com-
plexity which is mainly due to diversity of
neurosecretory fibres and tinctorial prop-
erties of the secretory material. The neuro-
secretory fibres in the organ branch and
swell repeatedly along their entire length.
The size of the bulbs varies extensively,
reaching at times a diameter of 30 /x. The
innumerable drops of varying sizes might
have prompted Kiihne to call this organ
the Tropfenkomplex. The homogeneous
pools of secretory material in some fibres
have the same staining property as those
present in the axons and in the soma of the
subesophageal ganglion. Most of these can
be traced back to their cell bodies in the
sub- and supraesophageal ganglia. The
secretory material in other axons stains
pink with PF, like the product present in
neurosecretory cells of the primary organ
of Schneider. But in the pools, the neuro-
secretory material is mostly in the granular
form.
The first organ of Schneider and the
Tropfenkomplex are covered by a thin
membrane, the neurilemma. Neurosecre-
tory cells are absent in the Tropfenkom-
plex. In sections, the organ presents a two
structured appearance (Plate 2, E, F). The
dorsal part is heavily loaded with secretion
bearing sacs, and axons of varying sizes.
The majority of them are arranged at right
angles to the neurilemmal sheath and thus*
appear as though hanging from the dorsal
wall of the ganglion. Thus, a kind of
palisade arrangement close to the blood
vessel was observed in the dorsal part of
the ganglion (Plate 2, F).
The ventral half, in contrast, contains
both secretion filled and ordinary axons.
The two types of secretory axons men-
tioned above are present in this region also.
The organization in this region is much
simpler because the axons run mostly par-
allel to each other. Thus, the palisade ap-
pearance found in the dorsal area is absent
here.
Amidst these structures, cells with a
nucleus and refractory cytoplasm extend-
ing into the numerous processes are pres-
ent. These are called the glial or Schwann
cells. A relatively great number of these
cells were found in the organ of Schneider
and in the main lobe of the Tropfenkom-
plex.
Changes in stainability of the
neurosecretory material
In the present study animals reared in
the laboratory have been used so that the
date of ecdysis preceding fixation was
known. This provides an accurate indica-
tion of variations in neurosecretion in the
course of the molt cycle.
From the second to the ninth stage the
total number of neurosecretory cells in
different ganglia during each intermolt
period was counted. The NSC were arbi-
trarily graded, as mentioned earlier, into
stage 1 (poor); stage 2 (middle) and
stage 3 (full). At the same time,Bthe
amount of stainable material present in
other regions such as neurosecretory axons
and the Tropfenkomplex was also divided
into these three stages.
In general, the cyclical accumulations
of the stainable material of each neuro-
secretory cell is different from that of other
nerve cells present in the same ganglion.
This type of asynchronous activity is more
common in type I neurosecretory cells of
aboral and abdominal groups. But the type
II cells in the cheliceral ganglia are mostly
synchronous in their accumulations. On
the basis of histochemical data collected
from more than 100 animals, the inter-
molt period in all stages can be divided
into an early, middle and late period. The
early period begins immediately after the
molt and ends by 24 hours later. During
this period, cell counts in 20 animals had
shown that 16-28% were poor; 55-69%
were medium and 18-33% were full with
secretory material. These animals also
showed medium quantities of secretory
material in the Tropfenkomplex and in
neurosecretory fibres in the neuropile
(fig. 5).
NEUROSECRETORY AND ENDOCRINE SYSTEMS IN A SPIDER
89
The middle period falls between two to
five days after the molt. Sixty animals
were observed during this period. Cell
counts in these animals had shown that
47-90% were poor; 2-45% were medium;
0-20% were full. The neurosecretory ma-
terial in the Tropfenkomplex of 25 animals
was poor; in ten it was medium and in
15 it was full (fig. 5). The neurosecretory
material in axons of 35 animals was poor
and in 15 it was medium. An exception
was found in five animals. In these 55% of
the cells were full, 44% were medium and
only 1% were poor. A corresponding high
degree of activity was also noticed in the
Tropfenkomplex and fibres in the neuro-
pile.
The late period starts by the sixth day
after the molt and in the majority of cases
ends before the fifteenth day. Cell counts
in 40 animals showed that 8-29% were
poor; 30-41% were medium; 52-68%
were full. The neurosecretory material in
axons and Tropfenkomplex of 30 animals
was full and only in ten it was poor
(fig. 5).
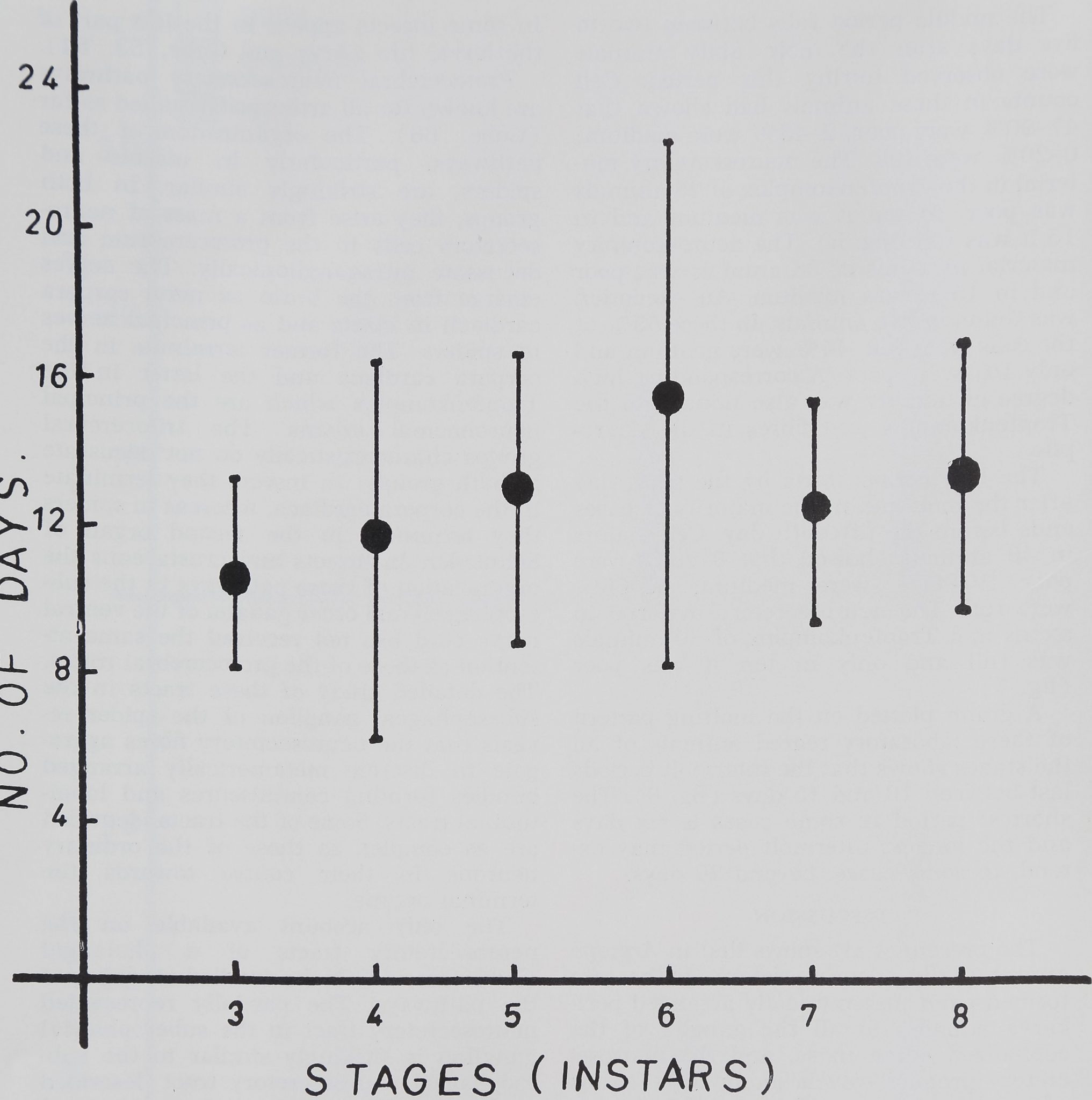
A graph plotted on the molting pattern
of these laboratory reared animals of all
the stages shows that the intermolt periods
last between 10 and 15 days (fig. 6). The
shortest period in some cases is six days
and the longest intermolt period may ex-
tend, in some cases, beyond 20 days.
DISCUSSION
The present study shows that in Argiope
aurantia the neurosecretory paths are
formed from metamerically arranged peri-
karya situated in all the ganglia of the
cephalized nerve mass, and the neurose-
cretory product travels along these axons
which terminate in neurohaemal organs.
The occurrence of neurosecretory cells in
all the postembryonic stages and the stage-
wise appearance of certain groups has also
been reported in other animals. In Opi-
liones (Naisse, ’59 ), the oral group can
be identified from the time of hatching,
whereas the lateral neurosecretory cells
were identified from the third larval stage.
Jones (’56) in Locusta; Sharan and Sahani
(’60) in Dysdercus; Khan and Fraser (’62)
in Periplaneta found protocerebral neuro-
secretory cells even during embryonic de-
velopment. The lateral neurosecretory cells
in some insects appear in the last part of
the larval life (Arvy and Gabe, ’53, ’54).
Protocerebral neurosecretory pathways
are known for all arthropods studied so far
(Gabe, ’66). The organization of these
pathways, particularly in insects and
spiders, are strikingly similar. In both
groups, they arise from a mass of neuro-
secretory cells in the protocerebrum and
decussate intraganglionically. The nerves
emerge from the brain as nervi corpora
cardiacii in insets and as principal nerves
in spiders. The former terminate in the
corpora cardiaca and the latter in the
Tropfenkomplex which are the principal
neurohaemal organs. The tritocerebral
groups characteristically do not decussate
in both groups: in insects they terminate
in the corpora cardiaca, whereas in spiders
they terminate in the second organ of
Schneider. In insects and crustaceans the
organization of these pathways in the sub-
esophageal and other ganglia of the ventral
nerve cord has not received the same at-
tention as those of the protocerebral tracts.
The detailed study of these tracts in the
subesophageal ganglion of the spider re-
veals that the neurosecretory fibres aggre-
gate in discrete metamerically arranged
bundles forming commissures and longi-
tudinal tracts. Some of the tracts identified
are as complex as those of the ordinary
neurons in their course towards the
terminal organs.
The only account available on the
neurosecretory tracts of a phalangid
(Juberthie, ’64) lacks detailed mapping of
the pathways. The partially represented
neurosecretory tract in the subesophageal
ganglion is strikingly similar to the sub-
esophageal neurosecretory tract described
in the spider. In spiders all free abdominal
ganglia have migrated forwards and fused
into a single, large subesophageal mass.
Perhaps, because of this anatomical spe-
cialization, the neurosecretory material
from several ganglia migrates into the
single large Tropfenkomplex. But in insects
and crustaceans which have a long ventral
nerve cord, other neurohaemal organs were
also reported. The median nerve neurohae-
mal organs in insects (Brady and Mad-
drell, ’67; Raabe and Ramade, ’67; Smalley,
’70) and pericardial organs in Crustacea
(Alexandrowicz, ’52, ’53) are some of
K. SASIRA BABU
Fig. 6 Length of molting cycle (third to eighth stages) in laboratory reared animals
of A. aurantia.
those present outside the cephalized ante-
rior masses.
The light microscopic anatomy of the
spider (Argiope) Tropfenkomplex provides
evidence as in other animal groups (Bern
and Hagadom, ’65; Gabe, ’66; Novak, ’66)
suggesting that this structure may be a
neurohaemal organ. The evidence is as
follows : ( 1 ) Secretion bearing axons enter
the organ from the protocerebral and sub-
esophageal ganglia. A blood sinus on the
dorsal side of the organ is present. (2)
The axons branch extensively and along
their course show swellings of various
dimensions. (3) These swellings or sacs
are filled with homogeneous colloidal ma-
terial which has the same staining proper-
ties as in the soma. (4) The Tropfenkom-
plex is enveloped by fibrous sheaths which
run into the organ. (5) The accumulations
histochemically show cyclical changes
which correspond to the stainability
NEUROSECRETORY AND ENDOCRINE SYSTEMS IN A SPIDER
91
changes in the perikarya. (6) Neuroglial
cells are present in the neurohaemal organ.
Further studies, especially at the electron
microscope level, are highly desirable.
Kühne (’59) stressed the absence of any
relationship between the morphology of
the neurosecretory perikaryons and the
period that lapsed since the last ecdysis.
In the present study two peaks of stain-
ability during the intermolt periods were
found. The first peak during the intermolt
period, regardless of stage or instar, is
suggested to be responsible for postmolt
development and differentiation of adult
characteristics. The second peak which
falls in the later part of the intermolt
period is prior to molting time. Since the
average time taken to complete a cycle of
ecdysis is between 10 to 15 days, the
second peak of activity comes between six
to ten days after the last molt. A corre-
sponding peak of accumulations in axons
and the Tropfenkomplex was observed. In
Opiliones, Naisse C59) reported two peaks
for the oral group and one peak for the
aboral group of neurosecretory cells. The
peak was closer to the time of ecdysis, and
thus, it was linked to the phenomenon of
molting. The stainability of the perikarya
in the pars-intercerebralis of insects under-
goes cyclical changes in the course of each
larval intermolt period (Rehm, ’51). Her-
lant-Meewis and Paquet (’56) in Carausius,
and Steel and Harmsen (VI) in Rhodnius
described two peaks of stainability in the
fifth larval intermolt where the second
peak of secretion in the perikaryons was
before the next ecdysis. In Bombyx
(Bounhiol et al., ’53) there was only one
peak of secretion before the next ecdysis.
Thus, the peak period of staining before
the molt as presented in spiders may also
be related to inducement of molting. In
spiders one or more cells in each group of
neurosecretory cells in the brain and sub-
esophageal ganglion which show the rhy-
thm may be involved in the process of
molting. Eckert (’67, ’68), after elimina-
tion of the oral neurosecretory cells and
the stomodeal bridge, reports that there is
either delay or complete elimination of
molting in Coelotes. Since an oral group is
absent in Argiope, experiments designed to
eliminate other groups of N.S.C. in the
brain and subesophageal ganglion of this
spider might prove useful.
In Argiope aurantia, the females show
external indications of sexual characters
in the form of epigynum only after the
sixth molt. During this seventh stage, the
neurosecretory cells in the cheliceral gan-
glia make their first appearance. From this
stage, they grow in size and in the amount
of secretory material synthesized and in
the amount transported along the axons.
Thus from the seventh stage there is mor-
phological and functional development of
the female reproductive system which can
be correlated with the appearance and in-
creased stainability of the cheliceral neuro-
secretory cells. Maximal staining and
axonal transport was observed in these
cells during the ninth stage, during which
maturation of gonads occurs. Females de-
posit their eggs in cocoons at 20-25 days
after the ninth molt. A corresponding in-
crease of neurosecretory material in several
cells of the aboral, first and second leg
and abdominal groups was noticed. The
stainability and transport along the axons
of these type I cells also reaches its maxi-
mum while the females are gravid. Hence,
it is suggested that both, type I and type
II neurosecretory cells may play a role in
the process of reproduction. Since it is
dangerous to draw conclusions only from
the stainability of N.S.C. (for further dis-
cussion see Highnam ’65), the best other
evidence of activity is furnished by the
axonal transport of neurosecretory mate-
rial (Lea and Thomson, ’62).
Thus the present results confirm the
earlier report of Legendre (’59); Kühne
(’59) and Streble (’66.) In adult phalangids
(Naisse, ’59) the neurosecretory cells of
the oral, aboral and lateral groups which
are rich in the secretory product are linked
with reproductive functions. A similar re-
lationship between neurosecretory activity
and reproduction was reported in insects
(Bounhiol, Gabe and Arvy, ’53; Highnam,
’61; Hoffman, ’70).
According to Kühne (’59) after oviposi-
tion the neurosecretory cells became pro-
gressively impoverished of secretion as the
animals aged. But in the present study it
was found that maximal accumulation
continued until the time of death. The
laboratory-reared animals and some from
92
K. SASIRA BABU
the field, laid two or even three cocoons
with and without eggs. This may be re-
sponsible for the continued presence of
high stainability of the neurosecretory
cells during this stage.
ACKNOWLEDGMENTS
I wish to express my gratitude to Dr.
Peter N. Witt for his constant interest and
suggestions during the course of this work.
My thanks are also due to Dr. I. R. Haga-
dom for valuable discussion and to Prof.
David E. Davis for his interest. The as-
sistance of Dr. Frank Enders in rearing
animals is gratefully acknowledged. This
work has been carried out in the Research
Division of the North Carolina Department
of Mental Health and Department of
Zoology, N. C. State University, Raleigh,
during the tenure of a Senior NSF fellow-
ship to the author, and with partial sup-
port from the N. C. Department of Mental
Health and NSF grant GB-6246 to Dr.
Peter Witt.
LITERATURE CITED
Alexandrowicz, J. S. 1952 Notes on the nervous
system in the Stomatopoda. Puhbl. Stat. zool.
Napoli., 23: 201-412.
—— 1953 Nervous organs in the pericardial
cavity of the decapod Crustacea. J. Mar. Biol.
Assoc. U. K., 31: 563-580.
Arvy, Land M. Gabe 1953 Données histo-
physiologiques sur la neurosécrétion chez
quelques Ephemeropteres. La Cellule., 56;
203-222.
—— 1954 The pars intercerebralis-cardi-
acum-allatum system of some Plecoptera. Biol.
Bull., 106: 1-14.
Babu, K. S. 1965 Anatomy of the central ner-
vous system of arachnids. Zool. Jb., Anat., 82:
1-154.
Bern, H. A., and I. R. Hagadorn L965 Neuro-
secretion. In: Structure and Function in the
Nervous System of Invertebrates. Vol. 1. T. H.
Bullock and G. A. Horridge, eds. Freeman, San
Francisco, California, pp. 353—429.
Bounhiol, J. J., M. Gabe and L. Arvy 1953
Données histophysiologiques sur la neurosé-
crétion chez Bombyx mori L. endocrines. Bull,
biol. France Belgique., 87: 233—333.
Brady, J., and S. H. P. Maddrell 1967 Neuro-
haemal organs in the medial nervous system
of insects. Z. Zellforsch., 76; 389—404.
Eckert, M. 1967 Experimentelle Untersuchun-
gen zur Hâutungsphysiologie bei Spinnen.
Zool. Jb., Physiol., 73: 49-101.
——– 1968 Untersuchungen zur hormonalen
Hautungskontrolle bei Spinnen. Vestnik Cs.
spol. Zool (Acta soc. Zool. Bohemosla.), 32;
34-38.
Gabe, M. 1954 Emplacement et connexions des
cellules neurosécrétrices chez quelques Ara-
néides. C. R. Acad. Sci. Paris, 238: 1265-1267.
——- 1955 Données histologiques sur la
neurosécrétion chez les Arachnides. Arch. Anat.
micr., 44: 351-383.
——- 1966 Neurosecretion. Pergamon Press,
Oxford.
Herlant-Meewis, H., and L. Paquet 1956 Neuro-
sécrétion et mue chez Carausius morosus Br.
Ann. Sci. Nat. Zool., 18: 163-169.
Highnam, K. C. 1961 The histology of the neu-
rosecretory system of the adult female desert
locust Schistocerca gregaria. Quart. J. micr.
Sci., 102: 27-38.
——- 1965 Some aspects of neurosecretion
in arthropods. Zool. Jb. Physiol., 71: 558-582.
Hoffman, H. J. 1970 Neuro-endocrine control
of diapause and oocyte maturation in the beetle,
Pterostichus nigrita. J. Insect. Physiol., 16:
629-642.
Jones, B. 1956 Endocrine activity during insect
embryogenesis. J. Exp. Biol., 33: 174-185.
Juberthie, C. 1964 Récherches sur la Biologie
des opilions. Ann. Speleo., 19: 1-237.
Khan, T. R., and A. Fraser 1962 Neurosecretion
in the embryo and later stages of the cock-
roach, Periplaneta americana L. In: Neuro-
secretion H. Heller and R. B. Clark, eds. Aca-
demic Press, New York, pp. 349—369.
Kühne, H. 1959 Die neurosekretorischen Zellen
und der retrozerebrale neurendokrine Complex
von Spinnen (Araneae, Labidognatha ) unter
Bervicksichtigung einiger histologisch erkenn-
baren Verânderungen wâhrend des postem-
bryonalen Lebens. Zool. Ja. Anat., 77: 527—600.
Lea, A. O., and E. Thomsen 1962 Cycles in the
synthetic activity of the medical neurosecretory
cells of Calliphora erythrocephala and their
regulation. Mem. Soc. Endocr., 12: 345-347.
Legendre, R. 1954 Données anatomiques sur le
complexe neuro-endocrine rétrocérébral des
Aranéides. Ann. Sc. Nat. Zool., 16: 419-426.
——- 1956a Les éléments neurosécréteurs
de la masse nerveuse et leur cycle d’activité
chez les Araignées. C. R. Acad. Sci. Paris, 242:
2254-2256.
——- 1956b Sur L’origine embryologique et
la repartition metamérique des cellules neuro-
sécrétrices chez les Araignées. C. R. Acad. Sci.
Paris, 242: 2405-2407.
——- 1959 Contribution à l’étude du système
nerveux des Aranéides. Ann. Sc. nat. Zool., 1:
339-474.
——- 1964—1966 Morphologie et Développe-
ment des Chelicérates, Embryologie, Dévéloppe-
ment et Anatomie des Araneidea. Fortschritte
der Zoologie, 17: 238-271.
Levi, H. W. 1968 The spider genera Gea and
Argiope in America (Araneae: Araneidea).
Bull. Mus. Comp. Zool., 136: 319-352.
Naisse, J. 1959 Neurosécrétion et glandes endo-
crines chez les opilions. Arch. Biol., 70;
217-264.
Novak, V. J. A. 1966 Insect Hormones. Methuen
Co., Ltd., London.
Raabe, M., and F. Ramade 1967 Observation
sur L’ultrastructure des organes périsym-
NEUROSECRETORY AND ENDOCRINE SYSTEMS IN A SPIDER
93
pathiques des Phasmides. C. R. Acad. Sci., 264:
77-80.
Reed, C. F., P. N. Witt and M. B. Scarboro 1969
The orb web during the life of Argiope au-
rantia (Lucas). Develop. Psychobiol., 2: 120-
129.
Rehm, M. 1951 Die zeitliche Folge der Tâtig-
keitsrhythmen inkretorischer Organe von
Ephestia kuhniella wahrend der Metamorphose
und des Imaginallebens. Arch Entw. Mech.,
145: 205-248.
Schneider, A. 1887/1892 Système stomato-
gastrique des Aranéides. Tabl. Zool. Poitiers.,
2: 87-94.
Sharan, R. K., and L. Sahani 1960 Provisional
embryonic cuticles of Dysdercus cingulatus.
Ann. Entom. Soc. Amer., 53: 538-541.
Smalley, K. N. 1970 Median nerve neurosecre-
tory cells in the abdominal ganglia of the cock-
roach, Periplaneta americana. J. Insect.
Physiol., 16: 241-250.
Steel, C. G. H., and R. Harmsen 1971 Dynamics
of the neurosecretory system in the brain of
an insect, Rhodnius prolixus, during growth
and molting. Gen. Comp. Endocrinol., 17:
125-141.
Streble, H. 1966 Untersucliungen iiber das hor-
monale System der Spinnentiere (Chelicerata)
unter besonderer Berücksichtigung des “endo-
krinen Gewebes” der Spinnen (Araneae). Zool.
Jb. Physiol., 72: 157-234.
PLATE 1
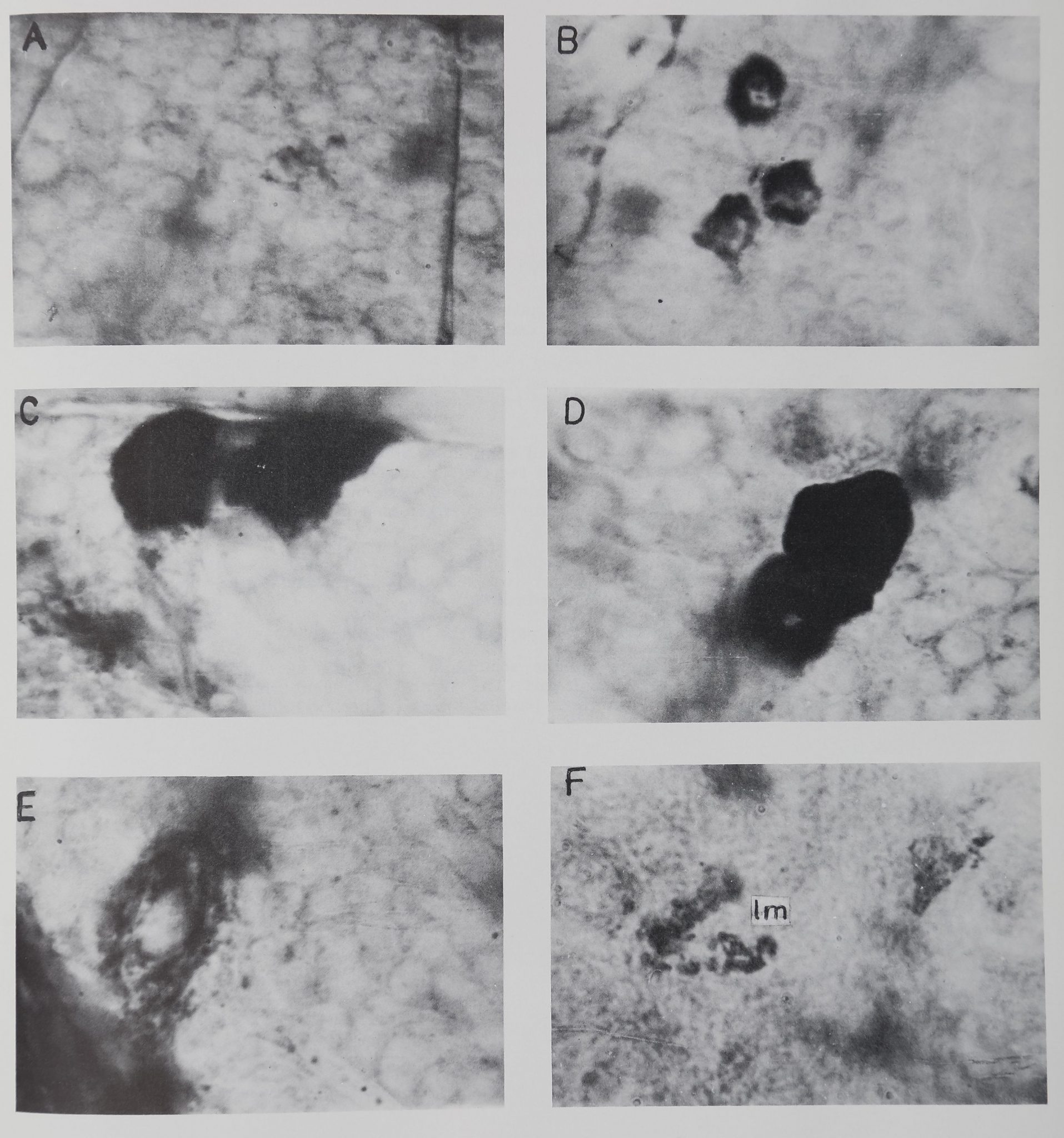
EXPLANATION OF FIGURES
A Type I neurosecretory cells. The two cells show very small quanti-
ties of the stainable material. Hence these are at stage I in syn-
thesis of the secretory material. ^ 1000.
B Type I neurosecretory cells. The three cells show medium quanti-
ties of the secretory material. Hence these are at stage II in their
secretory activity. || 1000.
C-D Type I neurosecretory cells (from ninth stage). The cells show
maximum quantities of secretory material. Hence these are at
stage III in the production of stainable material. C, aboral group
of cells. D, neurosecretory cells from the first ambulatory ganglion.
X 1000.
E Type II neurosecretory cell from cheliceral ganglion, x 1000.
F Densely stained and lobed areas (lm) in the perikaryon of large
nerve cells. These masses are PAS positive but when treated with
diastase they disappear completely. Hence the stained material
is most likely glycogen, x 1000.
94
PLATE 1
95
PLATE 2
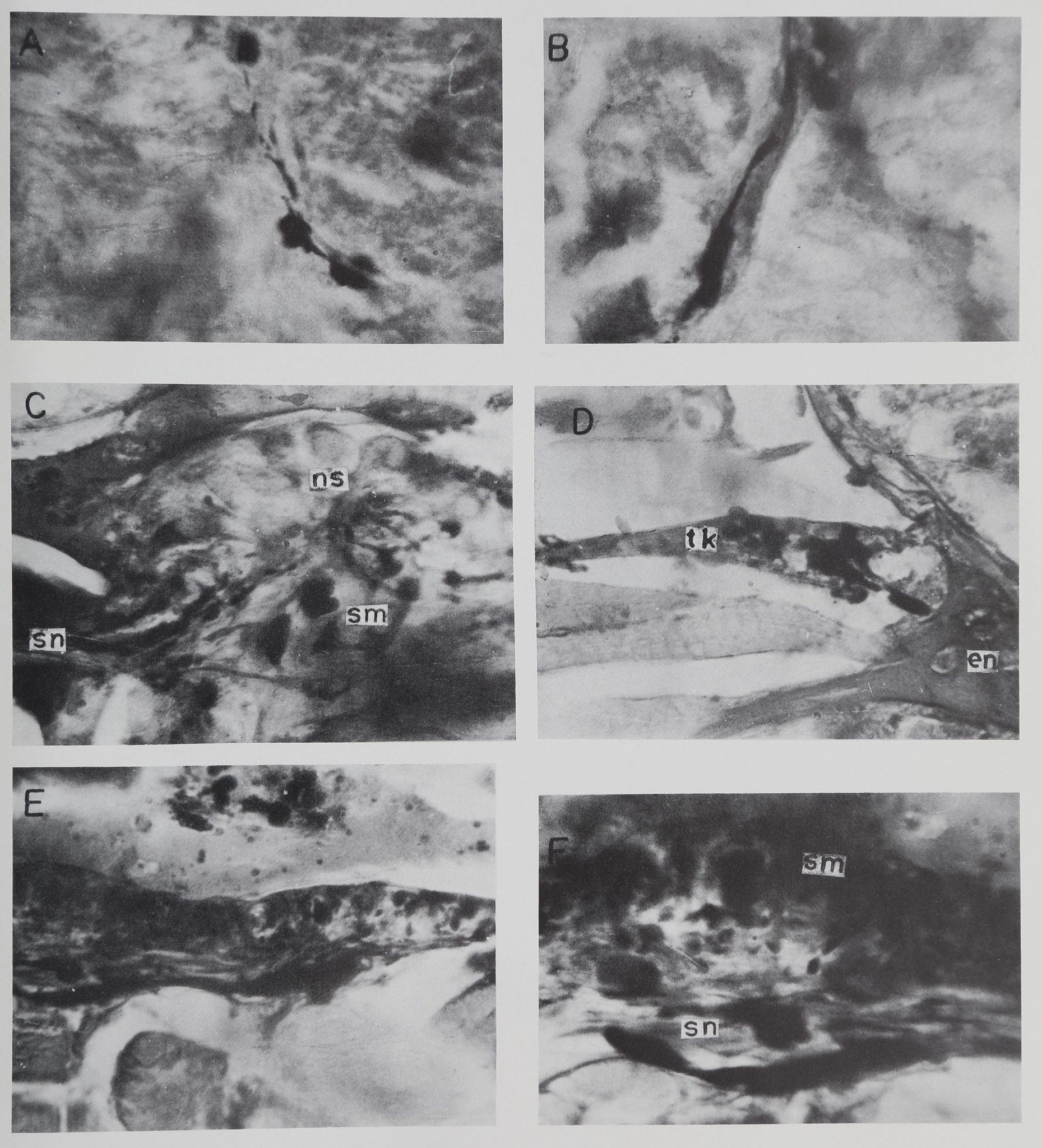
EXPLANATION OF FIGURES
A Pools of neurosecretory material within the neuropile. These fibres
arise from the neurosecretory cells of the first ambulatory ganglion.
X 1000.
B Extraganglionic course of a secretion filled axon on its way to the
Tropfenkomplex from neurosecretory cells of the first ambulatory
ganglion, m 1000.
C Sagittal section of first organ of Schneider. Note the nerve cells (ns)
in the upper half and pools of secretory material (sm) in the lower
half. Several secretion filled axons (sn) are present at the lower left
hand corner through which the Tropfenkomplex is connected with
the first organ of Schneider.
D Transverse section of the Tropfenkomplex (tk) at one of its lateral
extensions. Note the entry of two secretion filled axons into the
neurohaemal organ, en, Endosternite. x 400.
E Sagittal section in the middle part of the Tropfenkomplex. x 400.
F Tropfenkomplex. Note greater accumulation of the secretory ma-
terial (sm) in the dorsal part. The ventral part contains mostly
secretory fibres (sn) running parallel to each other, x 1000.
96
PLATE 2
97