Images Collection
View this article in Search Friendly Plain Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
Selection of Habitat by the Spider Argiope
aurantia Lucas (Araneidae)
FRUiK ENDERS
▼
Reprinted from
THE AMERICAN MIDLAND NATURALIST
Vol. 90, No. 1, July, 1973, pp. 47-55
University of Notre Dame Pres
Notre Dame, Indiana
Selection of Habitat by the Spider Argiope
aurantia Lucas (Araneidae)
FRANK ENDERS
North Carolina Department of Mental Health,
Division of Research> Raleigh 276111
Abstract : Visual search for the webs and egg sacs of Argiope aurantia
Lucas showed that it is most abundant in dense perennial vegetation.
This pattern of distribution was confirmed by release of the species in
selected plant communities: more spiders remained in densely vegetated
sites than in sparsely vegetated ones, both in the 2nd instar and in middle
immature instars; but adults and subadults left densely vegetated wood-
lands to build webs at the edge of those woods. It is suggested that
wind reduction is the significant stimulus to immatures, and light, to
adults.
Introduction
Argiope aurantia Lucas is among the commonest of spiders, locally
known as the “writing spider” due to the presence of a vertical white
mark in its orb web. This spider has been reported from a broad range
of habitats, including grasslands and woodlands (Fitch, 1963), and
from the edges of bodies of water to dry grassy hillsides (Levi, 1968).
The use by this spider in the daytime of a fixed wèb allows easy
determination of its location. The vertical mark of silk in the web, the
bright yellow and black markings and large size of the adult, the large
web of the adult and the exposed position in which the egg sacs are
placed — all contribute to make this spider easy to find by visual
search. Beating with a net, used by earlier workérs, actually collects
only a small fraction of the spiders of this species observed to be in an
area. In large part, this is due to the fact that the spider jumps out of
its web3 which is near the ground, when vegetation nearby is disturbed.
Here, I describe the population density of this species in the range
of habitats occupied in North Carolina, as determined by visual search.
By the release of this species in various plant communities, I tested the
idea that Argiope aurantia is restricted to stands of plants having a
particular physical structure.
Acknowledgments.—This research was in part supported by NSF Grant
GB-6246 to Dr. Peter N. Witt, and is a portion of a thesis carried out under his
guidance and submitted to the Graduate School of North Carolina State Uni-
versity in partial fulfillment of the requirements for the Ph.D. degree in zoology.
Dr. Herbert W. Levi confirmed my identification of the species mentioned in
this paper, and specimens have been deposited in the Museum of Comparative
Zoology. Dr. Donald C. Lowrie provided many helpful comments on this paper.
Distribution of Argiope aurantia among Plant Communities
Methods.—From 1968 to 1972 I spent several days a week in the
Raleigh-Durham area of North Carolina searching for Argiope
aurantia and other orb-weaving spiders. Searches were sometimes con-
47
The American Midland Naturalist
90(1)
ducted by beating the vegetation with a net, but, more often, visual
searches were made.
In February and May of 1971, I made systematic searches for the
egg sacs of Argiope aurantia in the largest accessible stands of the early
stages of old-field succession. I walked a minimum of 100 paces
through an area of uniform vegetation from a point of access, usually
a road. I walked toward any egg sac that I spotted and collected it.
I recorded the sort of vegetation that dominated the area searched, the
number of paces walked, and the number of egg sacs collected. This
gave an index to the relative abundance of Argiope aurantia.
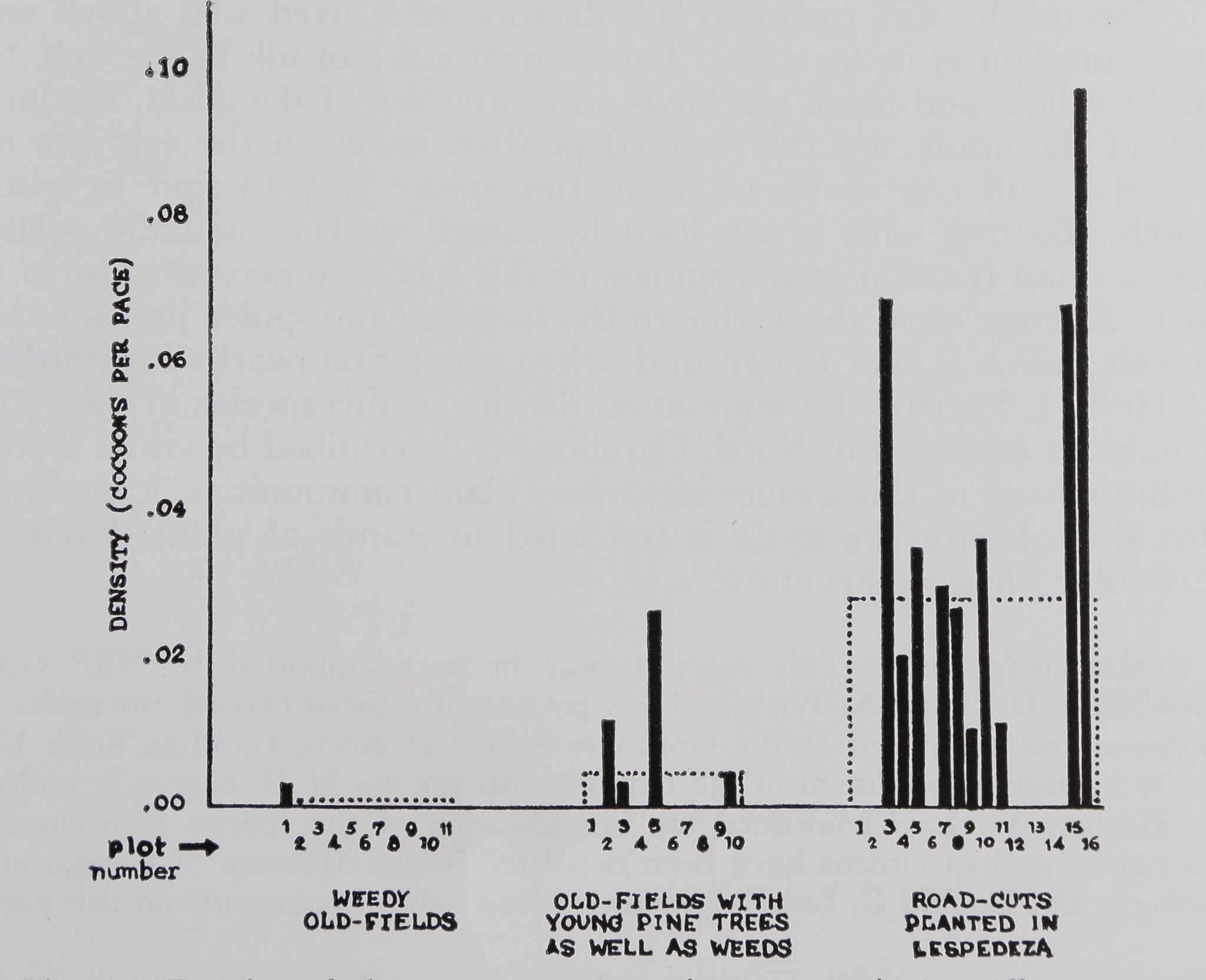
Results.—This spider was quite rare in old fields before trees and
shrubs invaded (Fig. 1). After shrubs were present, the spider reached
“moderate55 densities, i.e., levels of egg sac density at about the aver-
age (dotted line) for the middle group of plots in Figure 1. The levels
of density of webs that prevailed in those plots earlier in the year are
also considered moderate. The spider disappeared from the plant com-
munities of succession by the time the tree canopy closed over. This
spider reached “high55 densities in road cuts covered with sericea
lespedeza (Lespedeza cuneata). The density of webs counted on ran-
domly selected plots in these lespedeza areas ranged from 11.7/sq m
Fig. 1.—Density of the egg sacs of Argiope aurantia in different sorts of
plant communities. The dotted lines represent the total number of egg sacs
found in each sort of plant community, divided by the total number of paces
taken to collect the egg sacs. One pace is approximately 0.75 m in length. The
small bars above each number represent the density of egg sacs in particular
stands
1973
Enders: Spider Habitat Selection
49
in May of 1971 to 1.6/sq m in September 1970 (Enders, 1972). The
highest density of egg sacs reached in an area with no shrubs or trees
present was part of an old dump; that high density may be the result
of the disturbance of secondary succession.
My other observations show the same pattern of distribution of
Argiope aurantia as the systematic search for egg sacs. I was regularly
able to find low densities of immatures and adults of this species in
fields abandoned the previous year, provided the dead plants had not
been plowed under. But I never found this species in fields of annual
crops: soybean, sorghum, maize and tobacco. I also found it at low
densities in a cattail (Typha) marsh. Although I found no A. aurantia
in woodlands until 1971, in July of that year I found six animals in a
mixed hardwood-pine forest. This forest was adjacent to an extremely
high density population of the spider in lespedeza, the second black
bar from the right, in Figure 1. The density of the species in this
woodland was about a thousandfold less than in the neighboring
lespedeza area, which had been sampled by searching randomly
selected plots. Although I left the spiders in the woodland, I could
find neither spiders nor egg sacs there later in the year, despite repeated
searches of both the area near the ground and the canopy of the forest.
In coastal North Carolina (Beaufort and Cape Hatteras areas), I
found A. aurantia in moderate density in two places: (1) Baccharis
with Ammophila, where a sand dune bordered a salt marsh; and (2)
in a stand of Myrica and an unidentified grass, overgrown with Smilax.
Both stands were shrubs mixed with grass, as the stands in old-fields
which had moderate densities.
High densities of immatures were found in two places: (1) the
edge of thickets (Ilex and Myrica) bordering? a Juncus roemerianus
marsh; and (2) Myrica thickets, 2 m in modal height, with herbs
covering an (estimated) 15% of the stand. At the coast, I did not find
A. aurantia in stands of climax forest or in any stands of grasses that
lacked shrubs.
Everywhere I found A. aurantia quite regularly in areas disturbed
by man: gardens typically supported low densities, especially where
weeds were present. Weedy roadsides as far S as Hunter’s Island, S.C.,
supported populations at all levels of density. Moderate to high den-
sities were found wherever large stands of planted lespedeza were
present at the edge of four-lane highways. These included road cuts S
of Fayetteville, N of Winston-Salem and W of Asheville, N.C. This
species was observed on road cuts as early as the second summer that
vegetation was present.
A. aurantia was found in early stages of plant succession, even in
lst-year abandoned fields, but its highest densities were reached where
shrubs had already invaded the plant community. In woodlands it
occurred only as immatures at very low density, adjacent to an extreme-
ly high density population in lespedeza. The extreme high densities
recorded in less disturbed areas of vegetation approximated the usual
high densities in stands of lespedeza on road cuts.
50
The American Midland Naturalist
90(1)
Releases
Methods.—I collected egg sacs of A. aurantia in the winter of 1970-
71, and stored them in refrigerators. In May, after this species had
emerged out of doors, I placed the stored egg sacs at room tempera-
ture. When these spiders emerged, I spread them over the vegetation
near the ground in adjacent stands of plants at Clayton, N.C. These
included a soybean field (rows, 1 yard apart; average height of plants,
14 cm), a heavily shaded swampy hardwood-pine forest with dense
undergrowth, the weedy edge of the woodland with the soybean, and
a road covered with dense crabgrass {Digitaria sp.) about 15 cm tall.
Additionally, at Raleigh, N.C., fewer individuals were released in a
hardwood-pine forest, the edge of those woods, the bare ground of
a parklike stand of pines (Pinus taeda), a maize field and a ryegrass
field.
In June 1971, more spiders were collected from the lespedeza areas
and marked with model airplane paint. They were released in the
following sites at Raleigh: the hardwoods-pine forest, its edge and a
pine plantation with closed canopy, no understory and considerable
litter. These spiders were held in individual jars for about 28 hr after
capture and marking. The jars were then opened at the release point
in late afternoon and left on the ground. Any animal which did not
leave its jar by the next morning was excluded from further considera-
tion. Different color markings were used on spiders where releases
were made repeatedly or in adjacent areas.
In August of 1970 and 1971, large females were released at the
same hardwood-pine woodland and edge areas as in the June experi-
ments at Raleigh. A pine woods used had a very dense understory of
vines and was not the one used in June. These spiders seemed to be
within 1 instar of sexual maturity, judging from the form of the clavus
and the darkening of the front legs.
The day after all releases, the nearby area was searched for webs.
The number of marked spiders which remained to build webs was
recorded. However, any distinctively marked animal which turned up
after the first search was added to that number. Similarly, the max-
imum number of webs of the unmarked 2nd instars used was recorded,
even if it occurred a few days after the release. The 2nd instars had
been released into areas where no naturally occurring A. aurantia were
found. Naturally occurring animals could be distinguished because
they were about an instar further along in their development than the
releases.
Results.—The 2nd instars that were found remained within 2 m of
the point of release; the immatures of the 2nd release, within 4 m; and
the “adults,” generally within 7 m. Only the adults could be found
outside the plant community in which they had been released, in which
case they had traveled more than 30 m to leave the woodlands.
Fewer spiders of the 2nd instar remained in the sparse vegetation
of the soybean field, and fewer of the later instars remained in wood-
lands (Table 1). These two results are in sharp contrast to the con-
1973
Enders : Spider Habitat Selection
51
sistently high percentage which remained at the woodland edge. In
addition to the results presented in the table, 2nd instar spiders
remained in the ryegrass field (planted the previous autumn), but not
in this year’s maize, or in the parklike stand of pines.
That the 2nd instar remains wherever dense vegetation is present
was confirmed by the fact that release of 6000 2nd instars in the same
soybean field in July resulted in at least 400 present on orb webs
(estimated by counting webs in a sample of the field). At this time,
the soybean had reached a height of about 30 cm, with considerable
density of vegetation.
The late instars do not remain in deciduous woodlands : except for
one which built in the canopy of the forest, all left the mixed hard-
wood-pine forest on the night of release or the next night. Nine of
this group were found that had moved from woodland release point
to the edge of the woods, a minimum of 30 m. Of these, four were
not found until the 2nd day after release. One animal climbed to the
top of the hardwood-pine forest and remained 8 m above the ground
for about a week. This species had never been reported to build its
web at so great an elevation. The fact that some adults remained in
the pine woodland may be related to the fact that some large trees had
fallen there — the webs of the spiders which remained there were not
in heavy shade, as were the webs built in the hardwood-pine woodland.
In cases where spiders left release points, no predation on the
spiders was ever observed. Also, once the spiders built webs, preda-
tion would result in an empty web: an unusual rate of appearance of
empty webs (or unusual rate of mortality of spiders) was not observed
in the groups of spiders which remained at release points. Instead,
upon release spiders could be regularly observed walking (on vegeta-
tion, ground or silk) or “ballooning” (Kaston, 1948) away from the
release sites. When the tall grass was mowed where the “edge” re-
leases of August 1970 had built webs, many spiders moved the ‘20 m
to unmowed areas. Otherwise, animals which once built webs, includ-
ing 2nd instar spiders, were found regularly at or near the same sites
for several weeks after release. During the releases reported above, no
Table 1.—Per cent of Argiope aurantiaspiders which remained in various
___________________________plant communities after being released there______________________________________________
Plant community Month Instar Hardwood- pine forest Pine forest Edge of hardwood- pine forest Dense crabgrass Soybean field
May 2nd 14% 12% 14% 0.5%
(3/22) (5/42) (5/36) (2/384)
June 4th-7th 10% 30% 37%
(2/20) (7/23) (7/19)
August 8 th-11th 9% 24% 74%
(5/54)* (4/17) (17/23)
# All five which remained in woodland left the 2nd night after r clcase
IiiK American Midland Naturalist
90(1)
immatures were found at any distance from the point of release. How-
ever, on one occasion several marked 6th instar immatures were found
again about 10 m away from their point of release. This was after an
abortive attempt to demonstrate aggregation in which an area of an
old field had vegetation removed except for one stem every 0.5 m,
an area 5 m x 5 m. These observations support the idea that the dis-
appearance of released spiders was due not to unusually high preda-
tion^ but to their dispersal from areas which they “judged” to be
unsuitable.
Discussion
Other authors have reported Argiope aurantia from the savannah
and early serai habitats in which I found this species (Kaston, 1948;
Lowrie, 1948; Muma and Muma, 1949; Barnes, 1953; Barnes and
Barnes, 1955; Fitch, 1963; Berry, 1967; Levi, 1968). When earlier
workers attempted to take spiders in a regular manner from the dif-
ferent stages of plant succession, not many specimens of this species
were taken (Lowrie, 1948; Berry, 1967). Nor have summaries of
museum collection data by Kaston (1948) and Levi (1968) provided
a clear, consistent statement of the occurrence and relative abundance
of A. aurantia in different sorts of fields.
Fitch (1963) stated that A. aurantia “is more tolerant of shade
than A. trifasciata, but less tolerant of exposed situations with sparse
vegetation.’5 My own observations of A. trifasciata (Forskal) indicate
that this species does occur at slightly earlier stages of succession, on
the average, than A. aurantia. Both my observations of habitats occu-
pied by A. aurantia and my releases of that species support Fitch’s
statements. Therefore, Fitch (1963) and I disagree with Kaston
(1948), who did not note any difference between these two species in
the range of habitats occupied, and with Levi (1968), who suspected
that A. trifasciata occurred in drier habitats. The data gathered by
Lowrie (1958) and Berry (1967 ) are insufficient to determine the rela-
tive habitat distribution of these two species; in fact, beating with a net
is an inadequate method for these two species, since one captures rela-
tively fewer A. aurantia than A. trifasciata, in areas where the densi-
ties of each have been estimated by the visual search of randomly
selected plots. Release of marked A. trifasciata in selected habitats
can neatly demonstrate any difference between the two species in the
type of habitat that is acceptable.
My releases of A. aurantia demonstrate that this species actively
selects certain habitats, characterized by dense vegetation near the
ground that is not heavily shaded in late summer. No pattern of
choice of particular species of plants was found. The range of habi-
tats thus chosen corresponds to that described by Fitch (1963) and
found by my visual search for the species.
The maximum recovery rates of released spiders reported here,
14%, 37% and 74% at different instars, may be related to the (differ-
ing) abilities of spiders (of different sizes) to disperse by “flying55 on
1973
Enders: Spider Habitat Selection
53
silk (ballooning, Kaston, 1948). Adult A. aurantia cannot balloon;
I have seen middle instars balloon horizontally; 2nd instars can balloon
vertically by using very slight thermal air currents. If a spider bal-
loons only a few meters, it becomes virtually unfindable for me. More-
over, spiders which balloon vertically can travel hundreds of kilometers,
since ballooning spiders have been collected several kilometers above
the earth’s surface (Glick, 1939). Moreover, the fact that this species
normally disperses from the location of the egg sacs in the springtime
can explain the very low fraction of 2nd instars which remained even
at the most densely vegetated sites of release. Due to the natural
occurrence of large numbers of egg sacs of A. aurantia in areas of
lespedeza, I could not conduct releases in such areas, and I could not
make observations there of dispersal from single egg sacs. However,
observations of the densities of webs of 2nd instars under egg sacs in
lespedeza suggested that dispersal under natural conditions approxi-
mated that observed during the releases. There was no marked clump-
ing of the webs under the egg sacs, while high densities of webs, like
that within 2 m of release points, prevailed throughout the lespedeza.
Only the larger-sized spiders left woodlands, though in laboratory
gradients all stages are positively phototactic (Enders, 1972). It may
be that in later life a positive response to light is no longer held in
check by a negative response to wind. Enders (1972) has presented
some evidence that the apparent response to vegetation density is via
the presence or absence of wind. Fortunately, the spider’s negative
response to wind was at least partially separable from the other, con-
founded, microclimatic factors (temperature and humidity), since
the araneid spiders tested prefer dry places, in gradients of humidity
(Enders, 1972; see also Cherrett, 1964).
The results described in this paper support the idea of Duffey
(1966) that spider species select the structure of the habitat as well
as the microclimate of the habitat. The stimuli which seem to deter-
mine whether a habitat is acceptable to A. aurantia would ordinarily
be controlled by the structural characteristics of the habitat: vegeta-
tion density nearby, as well as at the actual web site, controls wind,
while light is controlled by the arrangement of the vegetation density
into strata which may shadow the ground. A. aurantia and most other
araneid spiders are very tolerant of desiccation and ordinary tempera-
ture extremes (Duffey, 1962). I, therefore, suggest that structural
characteristics of the environment are the primary influences upon
web site selection by members of the family Araneidae, while micro-
climate is of minor importance. In contrast, Riechert (1973) suggests
that thermal budget is of prime importance for a desert spider of the
family Agelenidae. However, (1) the species of agelenid may be a
special case, since temperature must be of importance to any animal in
a hot desert, and (2) agelenids and araneids are adapted to micro-
habitats having much different exposure to> desiccation. Agelenids’
webs occur near the ground or inside herbage, while araneids build
aerial webs in spaces exposed to the elements (Duffey, 1962). In fact,
The American Midland Naturalist
90(1)
54
an agelenid species I tested showed a preference for the moist end of
humidity gradients, while araneids preferred the drier end (Enders,
1972).
Only in “edge” habitats did A. aurantia reach high densities of
population: lespedeza, young bay berry (Myrica) and mixed salt
marsh-coastal scrub. All these areas had a vegetation density that
would induce settling by released 2nd instars, and yet none were
shaded enough to cause adults to leave. Thus, each area had several
years for colonization by ballooning A. aurantia and for the local
population to build up, since about 13% of the 2nd instars remain near
the place of emergence, in vegetation that is suitably dense (Table 1).
In this manner, this species is favored by ecotonal situations, even
though it can survive in the early stages of plant succession if no eco-
tone is present. For more than a century, the edges of roads have
provided a suitable man-made ecotonal habitat (Wilder, 1873; Bilsing,
1920), while before that the edges of bodies of water must have been
the prime ecotone available. Though the excellent dispersal abilities of
A. aurantia (Glick, 1939) must allow it to reach areas hundreds of
kilometers away from the source, today’s road system provides this
spider a ubiquitous source for any large area of suitable perennial
vegetation that develops during plant succession. As there are a con-
siderable number of species of spiders that utilize similar successional
and ecotonal habitats (Berry, 1967), there are implications for the
management of habitat diversity to reduce insect numbers (de Loach,
1970). Hedgerows and early planting of crops should favor A. auran-
tia, while monoculture and the manicuring of road edges should reduce
its numbers considerably.
References
Barnes, R. D. 1953. The ecological distribution of spiders in non-forest mari-
time communities at Beaufort, North Carolina. Ecol. Monogr., 23:
315-337.
——-and B. M. Barnes. 1955. The spider population of the abstract broom
sedge community of the southeastern piedmont. Ecology, 36:658-666.
Berry, J. W. 1967. The distributional ecology of spiders in the old-field succes-
sion of the Piedmont region of North Carolina. Ph.D. Thesis, Duke
University, Durham, N.C. 259 p.
Bilsing, S. W. 1920. Quantitative studies in the food of spiders. Ohio /. Sci
20:215-260.
Cherrëtt, J. M. 1964. The distribution of spiders on the Moor House National
Nature Reserve, Westmoreland. /. Anim. EcoL> 33:27-48.
Duffey, E. 1962. A population study of spiders in limestone grassland, the
field-layer fauna. Oikos, 13:15-34.
——-. 1966. Spider ecology and habitat structure (Arach., Araneae). Senck-
enbergiana Biol., 47:45-49.
Enders, F. 1972. Web site selection by Argiope aurantia Lucas and other orb
weaving spiders (Araneidae), Ph.D. Thesis, North Carolina State Uni-
versity, Raleigh, N.C. 168 p.
Fitch, H. A. 1963. Spiders of the University of Kansas Natural History Reser-
vation and Rockefeller Experimental Tract. Unit). Kans. Mus, Natur.
Hist, Mise. Publ.y 33:1-202.
1973
Enders: Spider Habitat Selection
55
G lick, P. A. 1939. The distribution of insects, spiders and mites in the air.
U. S. Dep. Agr. Tech. Bull., 673:1-150.
Kaston, B. J. 1948. Spiders of Connecticut. Conn. State Geol. Natur. Hist.
Surv. Bull., 70:1-874.
Levi, H. W. 1968. The spider genera Gea and Argiope in America (Araneae:
Araneidae). Bull. Mus. Comp. Zool., 136:319-352.
Loach, G. J. de. 1970. The effect of habitat diversity on predation. Broc. Tall
Timbers Conf. on Ecological Anim. Control by Habitat Manage., 2:
222-241.
Lowrie, D. G. 1948. The ecological succession of spiders of the Chicago area.
Ecology, 29:334-351.
Muma, M. H. and K. E. Muma. 1949. Studies on a population of prairie
spiders. 76z<7., 30:485-503.
Rieghert, S. E. 1973. The patterns of Agelenopsis web distribution. /. Anim.
Ecol., in press.
Wilder, B. G. 1873. The habits and parasites of Epeira (Argiope) riparia,
with a note on the moulting of Nephila plumipes. Broc. Amer. Ass. Adv.
Sci., 22:257-263.
Submitted 5 July 1972
Accepted 14 November 1972