Images Collection
View this article in Search Friendly Plain Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
Reprinted fr°ni The Journal of Experimental Zoology
Vol. 175, No. 1, September 1970 © The Wistar Institute Press 1970
Structure and Function of Tarsal Sensilla in the Spider Araneus diadematus
RAINER F. FOELIX
North Carolina Department of Mental Health, Division of Research, Raleigh, North Carolina
ABSTRACT The tarsal hairs of the spider Araneus diadematus (Clerck) were examined by light and electron microscopy and for surface structures with a scanning electron microscope. Three different types of hairs could be found: (1) straight, sharp-pointed hairs, (2) serrated bristles, (3) curved, blunt-tipped hairs. The third type of hair shows all typical features of chêmosensitive hairs, such as multiple innervation and an open tip, and was not included in the present study. The first two types share the same histological characteristics of having the dendrite of one bipolar neuron attached proximally to the hair base. Also, most of the fine structural details, such as the presence of an extracellular space, a scolopale and a tubular body, correspond to tactile hairs as described in insects. However, in Araneus diadematus the nerve terminal has a three-lobed structure and seems to be fastened to the socket cuticle which is not known from other arthropods. The five or six serrated bristles at the tip of the tarsus, together with the middle hook, grasp the thread. This relationship was suggested by Nielsen (’31) and was verified by means of the scanning electron microscope. The importance of this mechanism for grasping the thread was demonstrated in ablation experiments with quantitative evaluation of changes in web geometry.
The hairy legs of an orb-web-building spider, i.e., Araneus diadematus Cl., play an essential role in web building: they serve the distribution of thread, placing it in exact position using information from existing structures (Reed et al., ’65; Reed, ’69). It must be assumed that such functions can only be performed if the legs are equipped with sense organs as well as mechanical devices of relatively high specialization. The most distal segment of the spiders leg, the tarsus, is of special interest because it makes the first direct contact with the environment. Numerous hairs cover the entire tarsus and its tip is equipped with three movable claws (2 main claws and 1 middle hook). Little attention has been given to the structure and possible function of the different hairs. Other than some early reports (Mclndoo, Tl; Schaxel, T9; Gossel, ’35) only the highly specialized trichobothria have been studied extensively (Gôrner, ’65; Gôrner and Andrews, ’69).
The purpose of this work is to describe the external morphology and the histology of the different sensilla on the tarsus of Araneus diadematus Cl. and determine their possible function. Most hairs on spi
der legs are believed to be tactile in function (Comstock, ’48) but there is no morphological or experimental evidence to support this hypothesis. Nielsen (’31) suggested that certain bristles act in opposition to the middle hook in order to grasp the silk thread. In the present study an ablation experiment was conducted to test the influence of these bristles on web building.
Spider hairs, as well as other arthropod hairs, should be more precisely termed “sensilla” (Barth, ’69) but both terms are commonly used.
MATERIAL AND METHODS
(a) Structure. Whole mounts of tarsi of all legs of Araneus diadematus were studied with the light microscope in order to determine distribution and surface structure of different bristles. Usually exuviae were used rather than fixed material. The scanning electron microscope “Stereoscan” (Cambridge Sci. Instr.) was of special value for investigating details in the surface structure. Especially the problem of how the thread is actually running through claws and bristles could be solved with the scanning electron micro-
J. Exp. Zool., 175: 99-124.
99
100
RAINER F. FOELIX
scope (SEM). For that purpose spiders were anesthetized in their web with CO2, The hub region with the spider was transferred to a copper-ring, which was glued on top of the specimen carrier for the SEM. This preparation was kept in the refrigerator (~ 20°C) in order to immobilize it in its original position before coating and examining in the SEM. For technical reasons only very small spiders could be used.
For histological investigations legs were removed from C02-anesthetized spiders, placed in cold buffered, 5% glutaraldehyde (Millonig, ’61 ; Sabatini et al., ’63) and perforated near points of interest for better penetration of the fixatives. After 8-12 hours they were washed in several changes of Millonig’s buffer (pH 7.2), post-fixed in buffered 1% 0s04 for two hours, dehydrated in graded alcohol series and embedded in Epon, Epon-Araldite or Durcupan ACM over propylene oxide. Before embedding some tarsi were dissected under a microscope and the different bristles were separated from each other. After re-orientation of the flat-embedded material, blocks were cut for electron microscopy on a Sor-vall MT 1 or MT 2 ultra-microtome with glass-knives, or occasionally, with a diamond knife. Parlodion-coated 100—200 mesh grids were usually used to pick up silver or light-golden sections. The staining procedures were the following : Uranyl-acetate in 50% ethanol for one hour and lead citrate (Venable and Coggeshall, ’65) for three to five minutes. Stained sections were examined in a Zeiss 9A electron microscope.
For light microscopy sections 1-3 ^ thick were cut from the same material on a normal Spencer rotary microtome, equipped with a special adapter for glass knives. Sections were stained with azure B- methylene blue (Richardson et al., ’60).
(b) Function. Subadult female Ara-neus diadematus were kept in open aluminum frames in the laboratory (Witt, ’56), where they build a web almost every day. The function of certain claws or bristles in web building was tested in ablation experiments. Removal was performed under a dissection microscope at a magnification of 40 X by means of fine watch maker’s forceps on briefly CCVanesthetized spiders.
At least three control webs of each spider built immediately before operation and as many webs as could possibly be obtained after operation (2-12) were recorded photographically. The negatives were then projected to the original size of the webs and measured at selected points (Reed et al., ’65). A computer program calculated such measurements as total thread length, web proportions and regularity of radial angles and spiral spacing from the raw data. For statistical analysis the mean values of each web measure before and after operation were compared by the use of the t-test (significant level of probability P<0.01). Each spider contributed one (mean) value for a certain measure, representing the average of all its control webs or post-operation webs respectively.
OBSERVATIONS
On the tarsus of Araneus diadematus (or any other member of the Araneidae) occur three different types of bristles (figs. 1, 2)^i(l) Straight, sharp-pointed hairs are the most common type. (2) Several curved, serrated bristles occur at the tip of the leg. (3) Slightly curved, blunt-tipped hairs are arranged in several rows between the straight hairs. The third type shows all features which are known to be characteristic for chemosensitive hairs in insects (multiple innervation, open tip, permeability to dyes) and therefore will be discussed in a later publication. A fourth bristle type, the trichobothrium, occurs on tibia and patella but not on the tarsus.
(1) Straight, sharp-pointed hair
Structure. This common hair covers the whole tarsus and is arranged in somewhat irregular rows. Hairs vary from 100 to 200 /x in length, (most frequently about 200 jti) and their diameter at the base is usually 8-10 ^ All bristles point towards the tip of the leg, forming an angle of 30° or less relative to the leg axis. At the base they insert obliquely in a slipper-shaped socket in the leg cuticle (fig. 3) and are freely suspended in a connecting membrane. The socket is 20 X 50 a in size and extends approximately 12 \x above the surface of the leg. In contrast to the scaly appearance of the leg cuticle, the surface of the socket is relatively
TARSAL HAIRS IN A WEB-BUILDING SPIDER
101
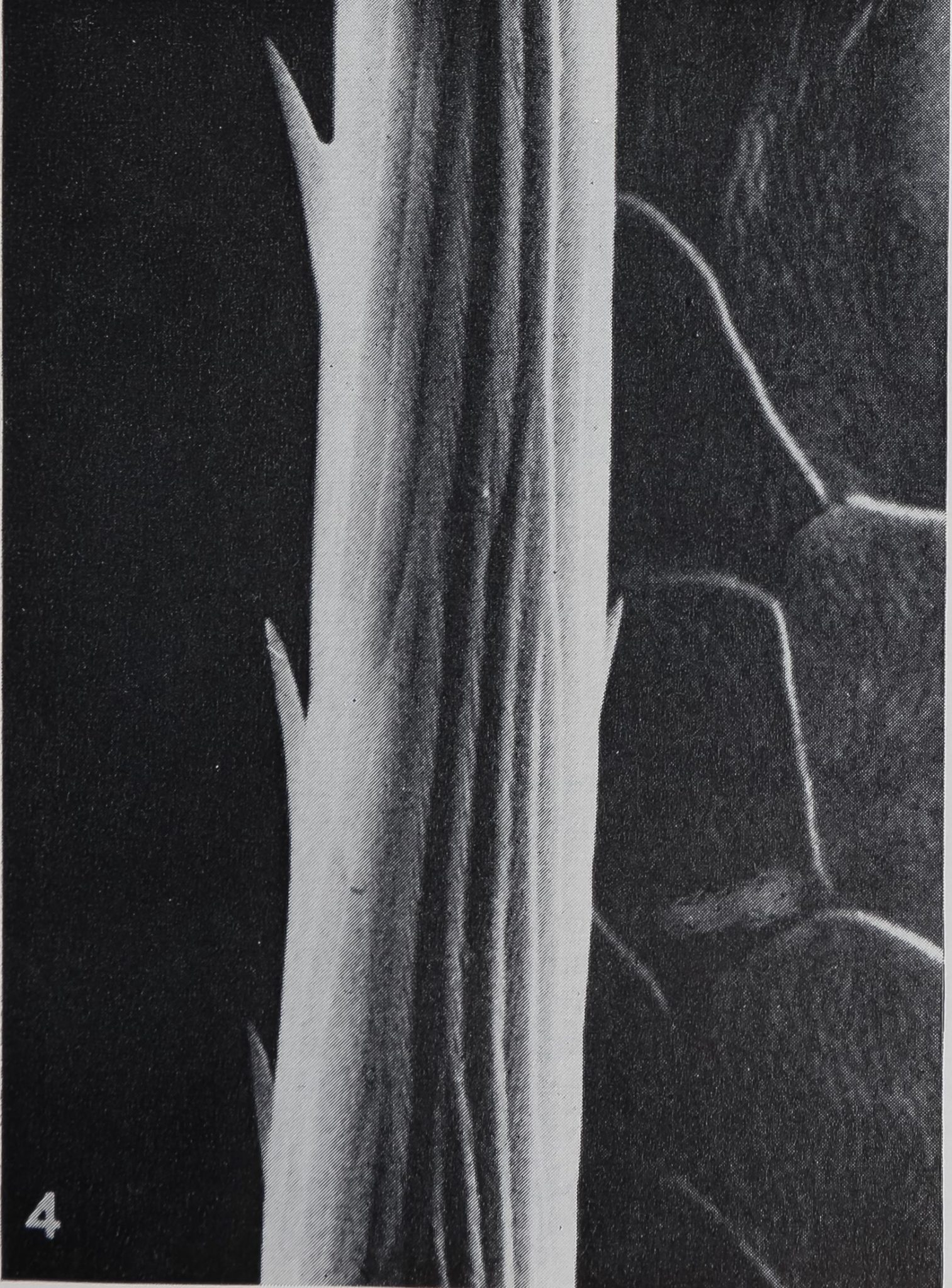
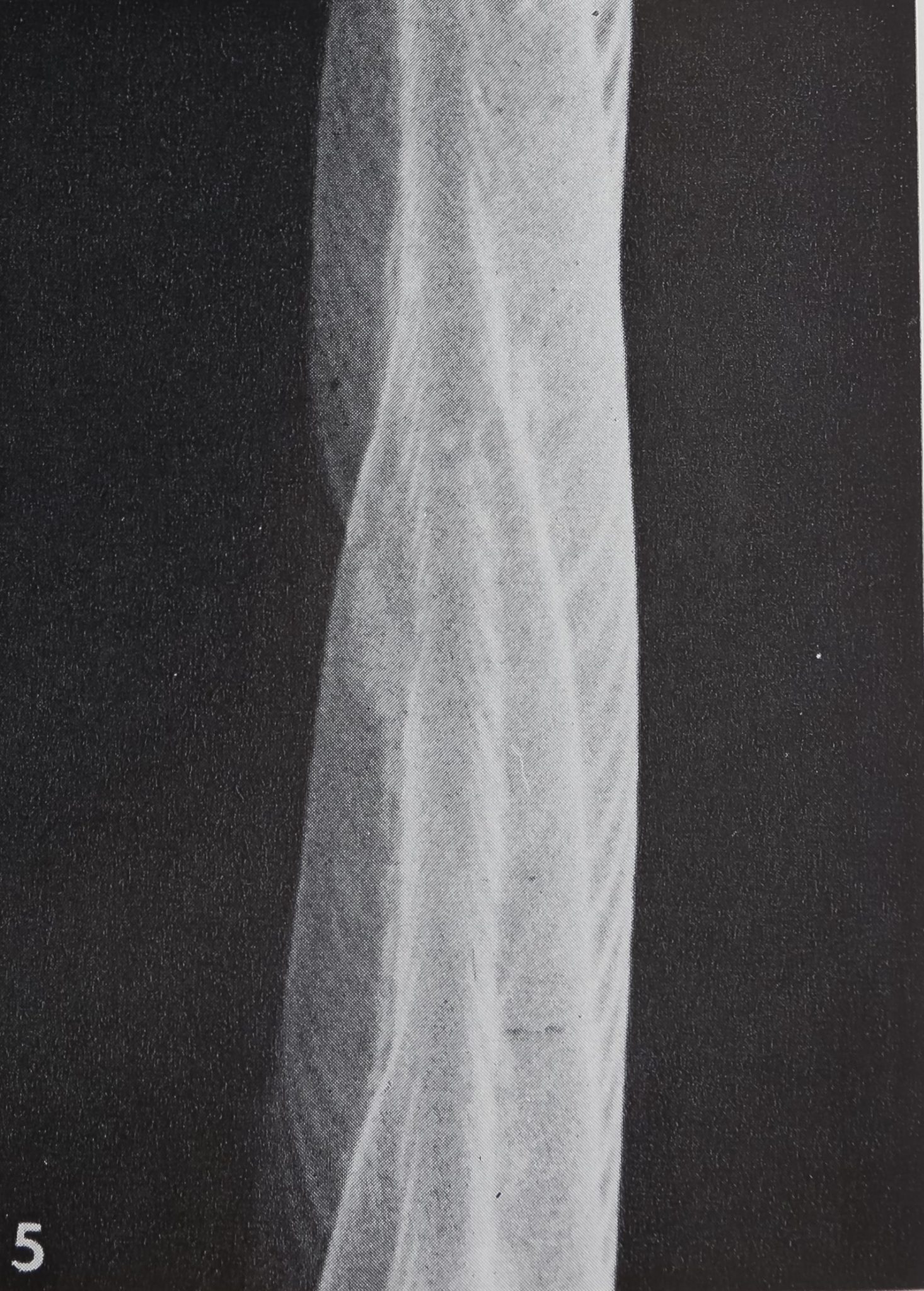
smooth; only the proximal slope bears fine longitudinal ribs. The surface structure of the hair can best be studied with the scanning electron microscope. Each hair has many longitudinal striae of 1.0-1.2 ^ in width, which fuse gradually and tend to spiral while running towards the tip (figs. 4, 5). These longitudinal striae consist of smaller ribs, being spaced at 0.15-0.2 p. The surface may also be covered by some small projections which are more pronounced on the hairs closest to the claws.
Under the light microscope these hairs possess an irregular lumen (fig. 2) of 3-4 in width, which ends shortly before reaching the fine pointed tip. The cuticle wall is approximately 3 /x thick and consists mainly of exocuticle (Barth, ’69). The connecting membrane at the base appears as a homogenous matrix with some cuticular fibers crossing it. Only the points of attachment between leg and hair cuticle stain deeply with methylene blue. This suggests the presence of resilin, a rubberlike protein (Weis-Fogh, ’60; Andersen and Weis-Fogh, ’64).
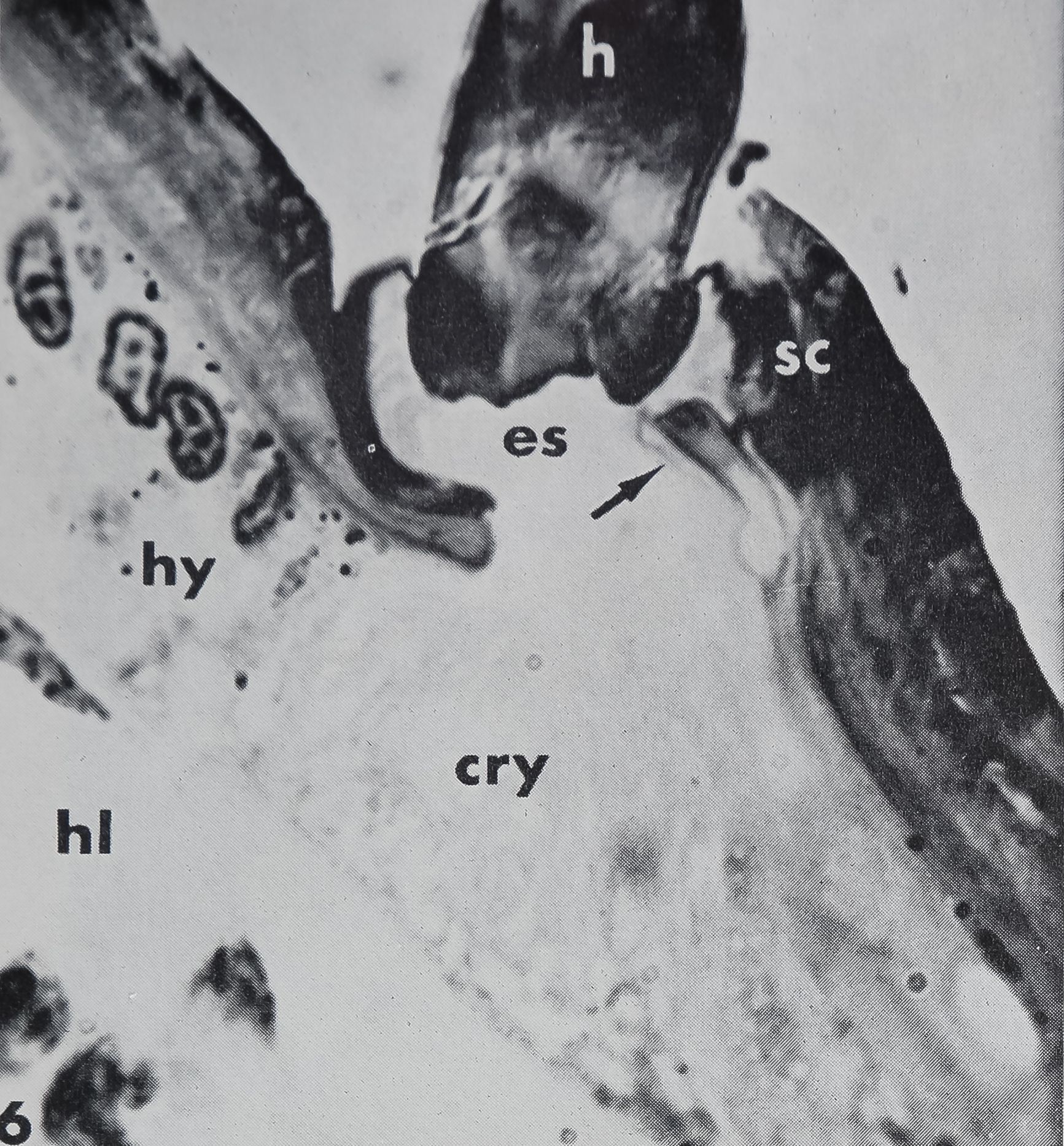
Every hair is supplied with one bipolar neuron embedded in the hypodermis (figs. 6, 7). The dendrite never enters the lumen but always ends at the proximal side of the hair base. The dendritic ending appears in longitudinal sections as a three lobed structure with a short constriction. Then the dendrite expands to an egg shape and narrows again, while running toward the neuron cell body, which lies almost 100 fi proximal to the hair base. Upon inspection of longitudinal sections no direct connection is usually visible between the dendrite and the hair itself (figs. 7, 8). However, a few favorable sections in the electron microscope reveal a fine extension of the middle lobe of the dendritic ending which is connected to the hair base (fig. 9). In tangential sections it can be seen that two fiber bundles forming a Y-shape establish contact between the lateral cuticle of the hair base and the dendritic ending (fig. 10). Electron micrographs of these fibers demonstrate that they do not belong to the dendrite but consist of some kind of extracellular material, possibly cuticular strands.
The reason why the three-lobed structure appears clearly in the light microscope is
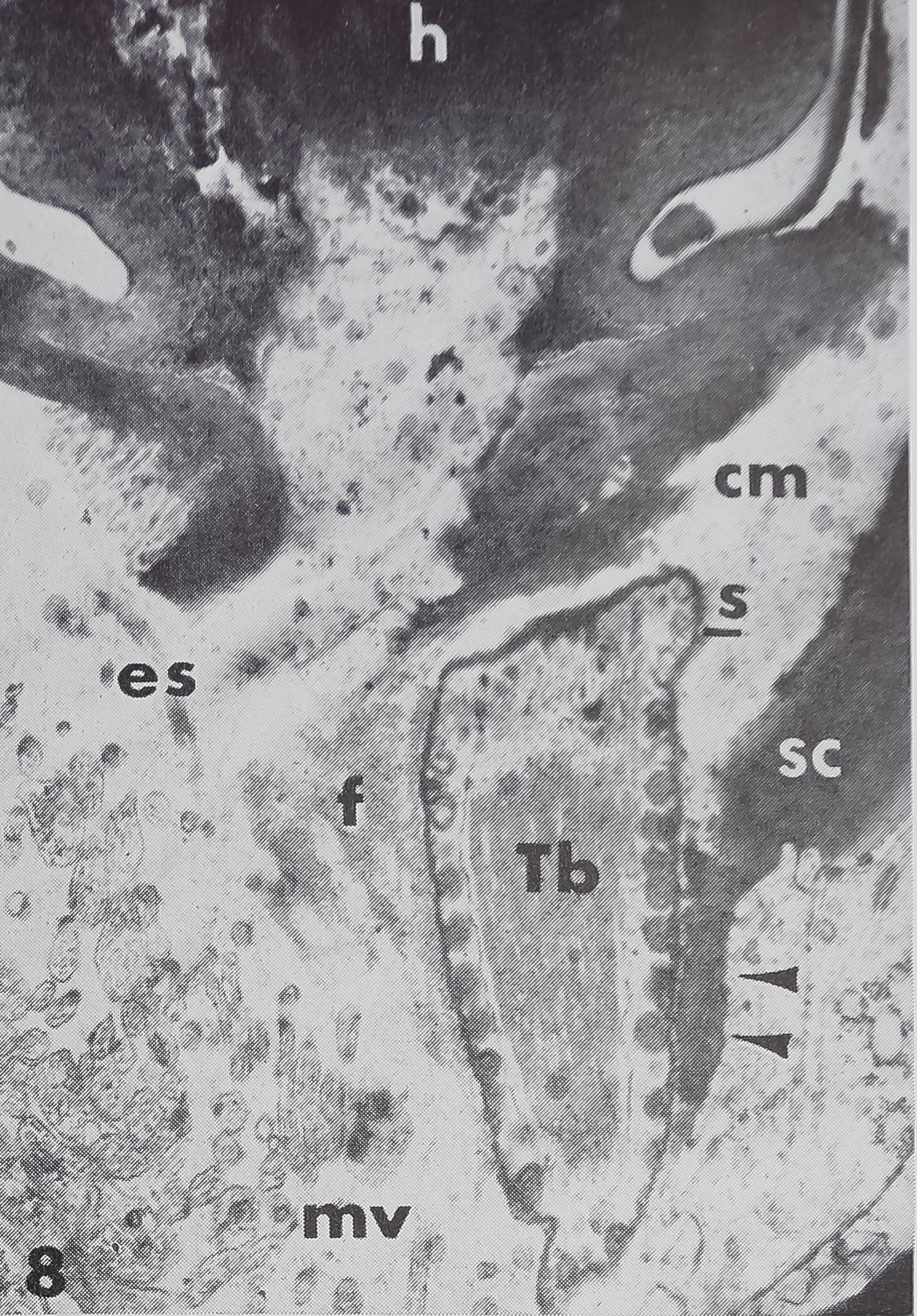
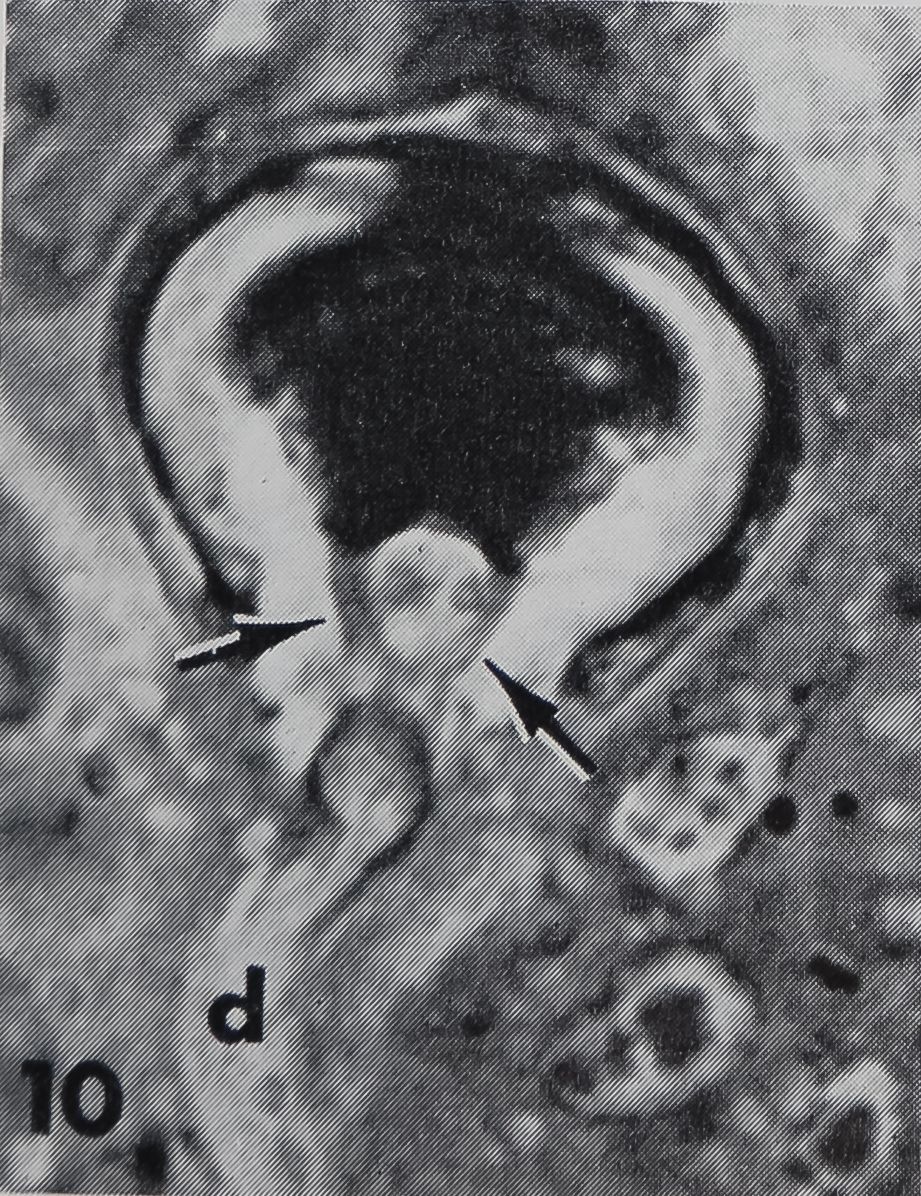
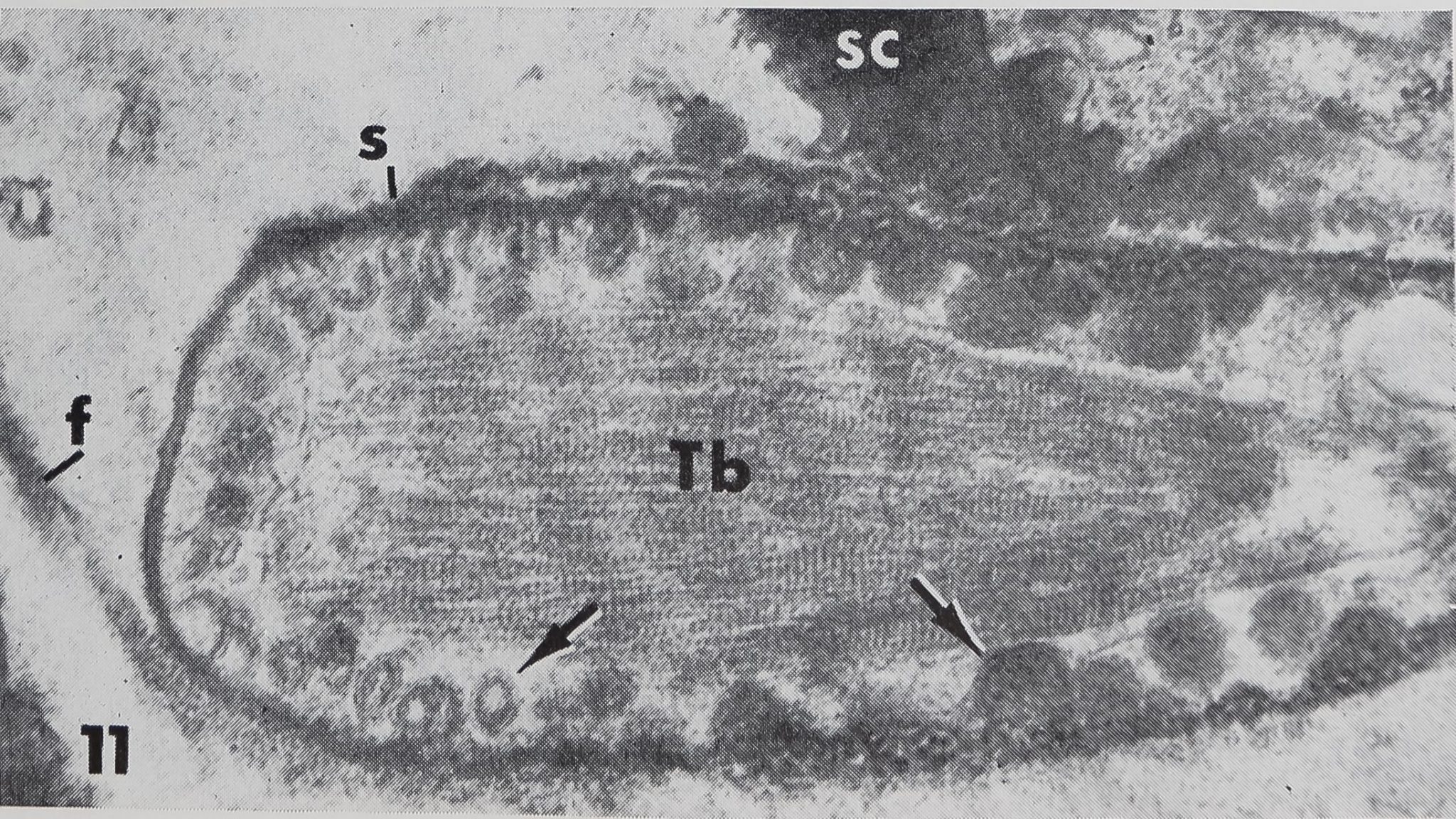
the presence of a “cuticular sheath” ( scolo-pale) which encloses the dendritic ending. Inside the three lobed structure are many tightly packed tubules (~ 170 Â diameter) associated with an electron-dense substance (figs. 8, 9, 11). These constitute the so-called “tubular body” as described in mechanoreceptive hairs of insects (Thurm, ’64). At higher magnification these tubules show a fine cross-striation, being spaced at about 250 Â (fig. 11). The tubular body does not extend distally into the last terminal channel to make contact with the hair base, but sends branches to all three lobes. The terminal channel contains only some microtubules and lacks any electron-dense substance. The three-lobed structure contains peripherally a layer of dense granules (0.15E- 0.23 ^ in diameter), but no other cell organelles are found in that outer portion of the dendrite. The granules possess a distinct membrane (160 — 190 À thick) and usually a dense core. In the very distal part of the dendritic ending however, the granules appear very light (figs. 8, 11) but are still membrane bounded. They are always closely associated with the inner side of the scolopale. The outer side of the scolopale seems to be fastened to a certain part of the socket cuticle; although there is no continuity between both structures, several points are in direct connection (fig. 11). Thus the three-lobed dendritic ending is attached distally to the movable hair base and proximally to the rigid socket cuticle. It was not possible to trace the dendrite all the way toward the cell body, and no basal bodies or other indications of a ciliary structure could be found. At least one, possibly two sheath cells enclose the dendrite, leaving the three-lobed ending free.
Just beyond the base of the hair is an extracellular space corresponding to the “tormogene vacuole” or “receptor lymph cavity” (Nicklaus et al., ’67) in insect sensilla. This space is filled with homogeneous fluid, presumably hemolymph; it sometimes contains blood cells. The cell underlying the hair base (tormogene cell ?) extends apically into many microvilli facing the fluid-filled cavity (fig. 8). In its basal part it contains a large amount of crystalline deposits (guanine ?).
102
RAINER F. FOELIX
The lumen of the hair appears hollow without any fluid in adult spiders. In the basal part some fibrous material, probably cuticular strands, may occur. In young spiderlings or freshly molted animals the hair lumen is filled with cell processes (probably of the trichogene cell) containing numerous microtubules.
Function. From its morphological similarity to the mechano-receptive hairs in the honey-bee (Thurm, ’64) and in the blow-fly (Richter, ’64) it seems likely that the straight, sharp-pointed hair is tactile in function. Because there is a connection between the hair base and the dendrite, any movement of the hair could be transmitted to the dendritic ending. In order to assure the tactile function of the straight, sharp-pointed hair, electro-physiological tests should be done.
( 2 ) Serrated bristle
Structure. In contrast to the slender and straight common hair on the tarsus, the bristles opposing the claws are thicker, distinctly curved, and possess many small teeth on their ventral side (fig. 12). Under the light microscope these bristles show a dark pigmentation and a bulbous base, whereas the other hairs are lighter pigmented and more constricted just above the point of attachment to the connecting membrane. The surface structure is similar to the straight hairs but less pronounced. Many transitional hairs occur on the tip of the tarsus. These are straight hairs possessing small teeth arranged over their entire surface or exclusively ventrally. To avoid confusions the term “serrated bristles” (“accessory claws” of Nielsen, ’31) is applied only to five or six dark pigmented bristles on the ventral side of the tip of the tarsus. The dimensions of the serrated bristles in adult animals range from 100 to 200 ^ in length and from 10 to 20 fi diameter at the base.
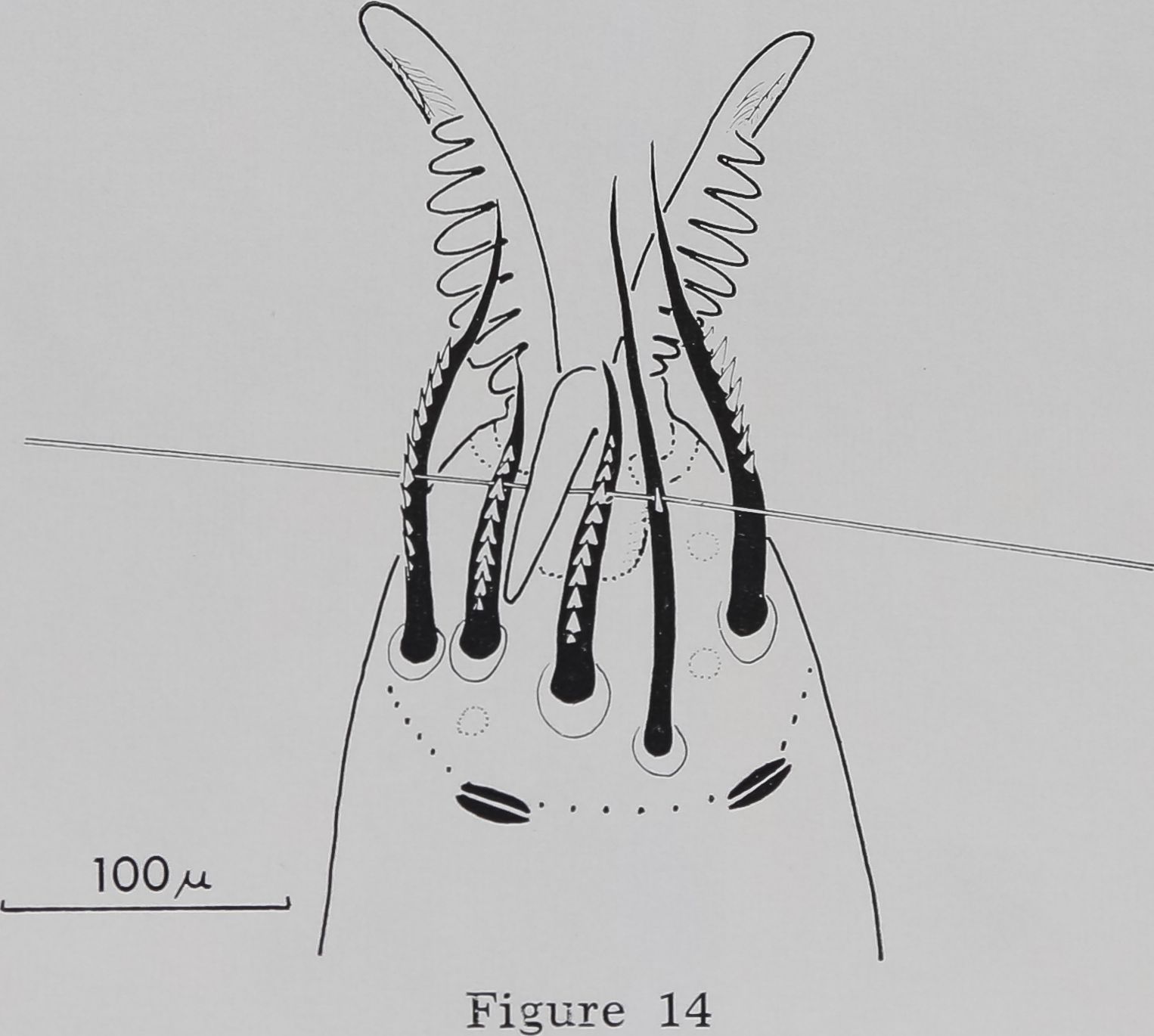
The serrated bristles are arranged in a semi-circle on the ventral side of the tip of the tarsus and oppose three movable claws (fig. 13). Two slit sensilla form the borderline at the proximal part of the tarsus (figs. 14, 17).
The distribution of the serrated bristles follows a pattern distinct for each pair of legs and is the same in both sexes. Leg 1
and leg 2 possess 5 serrated bristles which are arranged in the same pattern (fig. 14). Leg 3 has 5 and leg 4 has 6 serrated bristles each set in a pattern of its own: whereas leg 3 has a V-shaped arrangement, the bristles on leg 4 arise in a semi-circle with an additional thick bristle on the inner side (fig. 13). The thick bristle is less pigmented than the other serrated bristles and has only three small teeth distally; it is somewhat arbitrary to call it a “serrated bristle.” The tarsus of leg 4 deviates most from the other legs: it is the only tarsus with large spines on the ventral side. The most distal one of these spines is known as the “sustentaculum.” Many other bristles occur towards the tip and are clearly provided with teeth. They are not “proper” serrated bristles, because they are relatively light pigmented and have no relation to the claws. The large number of spines and bristles on the tarsus of leg 4 seems reasonable, since this leg is mainly involved in manipulating the thread during web building or wrapping the prey.
The arrangement of the serrated bristles and their structure with regard to length, degree of bending, and number of teeth is relatively invariant. Thus, it is possible to assign numbers to specific bristles for easier identification. It is most interesting that the middle hook always points between the same bristles: in leg 1 and 2 between bristle 3 and 4, in leg 3 and 4 between bristle 2 and 3 (numbers were given arbitrarily from the outside to the inside ).
All serrated bristles are innervated by one bipolar neuron in the same way as described for the common straight hair and possess a three-lobed dendritic ending which is proximally connected to the hair base.
Function. The constant position of the middle hook indicates a functional relation to the serrated bristles. Histologically they correspond completely to the common straight hair described above, thus indicating their tactile function. From their position one may conclude that they act in opposition to the claws when grasping the thread. Nielsen (’31) mentions that the middle hook is used mostly when holding on to the silk thread in the web (fig. 15).
TARSAL HAIRS IN A WEB-BUILDING SPIDER
103
He proposes that the little notches of the serrated bristles (“accessory claws”) could serve as an excellent device for the thread to be pushed against by the middle hook, thus providing a firm grip. He states however: “. . . unfortunately it has been impossible to ascertain this by direct observation.” Because the thickness of the thread is only 1-2 it is quite understandable that this relation is invisible under the normal light microscope. Wilson (’62) gives a diagram illustrating the same mechanism, but the source of his information is not clear. I tried to test Nielsen’s theory by means of the scanning electron microscope ( SEM).
The animal was anesthetized in its web with C02 and then kept in a refrigerator to immobilize it in its original state (see Material and Methods). Anesthetized (or dead) spiders drop out of their web when slightly moved; this demonstrates that grasping the thread is an active process and not merely a passive hanging. In order to get a stabile preparation, it was necessary to fasten the abdomen of the animal with a needle to the specimen carrier.
Figure 16 shows that Nielsen’s theory is correct. The silk thread is held by the middle hook and runs through the notches of several serrated bristles (fig. 14). The thread is easily released when the middle hook is lifted by the action of the M. levator pretarsi. Only occasionally the thread runs through the main claws and gets caught in the dentation of the combs.
After knowing the mechanism of grasping the thread it became important to determine whether the interaction between
middle hook and serrated bristles is essential for the spider in order to build a web. The easiest way to test this was to remove all serrated bristles on all legs (fig, 17) and observe the effect on web building.
Three control webs built by each of five spiders immediately before the operation were photographed (fig. 18) and compared with 38 post-operation webs (t-test; see Material and Methods).
The effect of the operation was obvious. Although all spiders were still able to build webs, the webs were smaller than controls and clearly irregular (fig. 19). The web measurements shown in table 1 differ significantly (t-test; p < 0.01) between webs built before and after operation.
The post-operation webs can be considered as less effective traps than the normal ones, because the catching area f spiral) was reduced more than 50% and the mesh width was increased about 40%. No significant change was noted in web proportions, such as width/length or position of the hub. These results indicate that the program of the web is still encoded in the central nervous system in the same way as before the operation^ but the “tools” (serrated bristles) are so damaged that the spider is no longer able to construct a regular web.
About one to two weeks after the operation three of the animals molted; they had regenerated all serrated bristles. The resulting “post-molt webs” showed a return to regularity (fig. 20). The mean values of 20 post-molt webs of three different spiders (n|3) are compared with nine control webs of the same spiders (n = 3)
TABLE 1
Effect of removal of all serrated bristles on web building
| Measure | Control (xc) (n,g5) | Op. (x0) (n = 5) | Effect | |
| 1. | Thread length(m) | 25.84 | 9.75 | decrease |
| 2. | Size of spiral (cm2) | 556.02 | 239.94 | decrease |
| 3. | Number of radii | 34.5 | 21.0 | decrease |
| 4. | Number of spiral turns 1 (West-North-East-South) | 31-32-36-43 | 17-12-16-23 | decrease |
| 5. | Mesh width 1 ( mm2 ) | 45.3 | 61.9 | larger |
| 6. | Spiral regularity 4 | 2 | 2 | |
| (West-North-East-South ) | 0.31-0.43-0.32-0.30 | 0.53-0.50-0.51-0.50 | less regular | |
| 7. | Angle regularity 1 | 2.03 | 6.62 | less regular |
Each figure represents the mean of five values; all differences (except one marked with 2) are statistically significant (t-test; P < 0.01). Note decrease in size and regularity of web when it was built by spiders without serrated bristles.
1 For definitions of measurements 4, 5, 6 and 7 see Reed et al. (’65) or Witt et al. (’68).
2 No significant difference in North direction (p, 0.08).
104
RAINER F. FOELIX
in table 2. These webs are still smaller and slightly less regular than before the operation, but there is a shift toward the control data. Because of the low sample size no statistical tests were performed on these data.
The ablation experiment indicates that only the intact mechanism of thread grasping between middle claws and serrated bristles as suggested by Nielsen (’31) permits the spider to build a regular web.
The most striking behavioral change was observed when a spider deprived of all serrated bristles was forced to climb a single thread. While normally a spider climbs the entire height of its frame (40 cm) in about two seconds, operated animals needed at least ten seconds. Slow motion pictures showed those spiders sliding down whenever they tried to grasp the thread. This observation corresponds to one of Nielsen (’31), where an Amauro-bius ferox (Amaurobiidae) which is not provided with serrated bristles by nature could not climb a vertical thread. Inside the web the operated animals could move quite well because the many transverse running threads apparently offer enough support when grasped with the middle hook alone.
It can also be proven experimentally that the two combed main claws play no important role in web building. Removal of all main claws on all legs causes no significant differences (P>0.01) in the web regularity compared to the normal webs (6 animals; mean values of 30 control webs were compared to 44 post-operation webs, table 3). Although there is a general decrease in size, number of radii
and spiral turns, the regularity of the web is not affected. The walking behavior in the web as well as climbing on a single thread is normal. This result is in accordance with the observation that the main claws are only occasionally used in grasping the thread. There is no other function known for the main claws.
(3) Claws
While working on the relationship between claws and bristles it seemed interesting to know if the claws are innervated. The external morphology and the arrangement of the two combed main claws and the middle hook were described by Frank (’57), but there is nothing known about their histology. Our studies show that the middle hook is supplied with five to six nerve fibers and the main claws only by one or two each (fig. 21). The dendrites are enclosed in a thick cuticular sheath (0.16 fx) that ends in the cuticle of the claws (figs. 22, 23). It seems that each scolopale contains two to three nerve processes and a small amount of fluid surrounding them. The ten double tubules seen in one of the nerve fibers (fig. 22) indicate that the dendrite might well be of a ciliary nature, although no sections of a ciliary region or a basal body were obtained.
The function of these nerve endings can only be surmised. They are probably a type of mechanoreceptor. For instance, they may respond to stress in the cuticle when the claws touch a solid substrate. In scorpions, the two corresponding claws may mediate a “scratch response” to vibrations
TABLE 2
Effect of the absence (X0) and regeneration (Xm) of serrated bristles on web building by three of the spiders evaluated in table 1
| Measure | Control (xc) N = 3 | Op. (x0) N = 3 | Molted (xm) N = 3 | |
| 1. | Thread length (m) | 29.89 | 12.36 | 19.54 |
| 2. | Size of spiral (cm2) | 640.09 | 312.22 | 448.44 |
| 3. | Number of radii | 38.5 | 23.4 | 27.9 |
| 4. | Number of spiral turns ( West-North-East-South ) | 35-36-38—46 | 19-13-20-25 | 24-18-28-34 |
| 5. | Mesh width (mm2) | 42.58 | 62.32 | 64.70 |
| 6. | Spiral regularity | 0.30-0.44-0.30-0.30 | 0.44-0.49-0.45-0.48 | 0.35-0.35-0.35-0.36 |
| 7. | Angle regularity | 1.86 | 5.02 | 3.32 |
Note the similarity between data in first and third column, if compared to second; only the webs in the second column were built without serrated bristles (compare with figs. 18, 19, 20).
Figures are mean values of nine control, 26 operational and 20 post-molt webs of three different spiders.
TARSAL HAIRS IN A WEB-BUILDING SPIDER
105
TABLE 3
Effect of removal of all main claws on web building 1
| Measure | Control (xc) (N = 6) | Op. (Xo)
(N = 6) |
|
| 1. | Thread length (m) | 24.82 | 17.95 |
| 2. | Size of spiral (cm2) | 531.76 | 385.73 |
| 3. | Number of radii | 32.1 | 28.6 |
| 4. | Number of spiral turns | 2 | 2 |
| ( W est-N or th-E ast-South ) | 32-32-37-45 | 26-22-30-35 | |
| 5. | Mesh width | 44.47 | 45.31 |
| 6. | Spiral regularity ( West-N orth-E ast-South ) | 0.39-0.51-0.39-0.37 | 0.40-0.45-0.38-0,38 |
| 7. | Angle regularity | 2.46 | 3.04 |
1 Compared to table 1 (effect of removal of serrated bristles) the changes in web geometry are small and insignificant.
2P, 0.02; all other values p > 0.05 (t-test).
of the substrate (Babu, personal communication).
(4) Slit sensilla
Several single slit sensilla occur on the tarsus of Aranens diadematus (figs. 14, 17), but no lyriform organ like the one on the metatarsus is found (fig. 24). The slit sensilla which are certainly mechanoreceptors were not included in this study because these sense organs have been investigated extensively by other authors (Barth, ’67; Barth and Deutsch-lànder, ’69 ).
DISCUSSION
Former studies on arachnid sensory hairs (Mclndoo, Tl; Schaxel, T9; Gossel, ’35) are based on whole mounts or paraffin sections, which, due to deficiencies in the technique, showed only few details. Recent advances in histological technique now permit a more accurate description of cuticular sensilla.
Gossel (’35) already noted two different “tactile hairs” on the legs of the spider Meta menardi (Araneidae), one inserting in an acute angle, the other one with a different socket, being more movable. His drawings show that these two types may correspond to the straight, sharp-pointed bristle and to the curved, blunt-tipped hair respectively. He pictures the first type with a nerve ending at the hair base and describes several neurons belonging to this hair, whereas in the second type a single sensory process enters the hair lumen. My preparations show clearly the opposite, namely, always one single bipolar neuron which is attached to the base of
the straight, sharp-pointed hair, whereas several nerve fibers enter the lumen of the curved hair. A single innervation is generally found in tactile hairs of arthropods, whereas most of the chemosensitive hairs are multi-innervated (Bullock and Hor-ridge, ’65). It seems therefore probable that Gossel’s second type of “tactile hair,” which corresponds to the curved blunt-tipped hair, is actually a chemosensitive bristle. The strongest argument in favor of that view is the fact that these hairs have an open tip, a characteristic feature of chemosensitive hairs in insects. A more detailed study of this type bristle is in preparation.
One of the points Gossel (’35) emphasizes is that the nerve ending is never differentiated. That means*a three-lobed dendritic ending or connecting fibers could not be observed in his preparations in contrast to mine. One explanation might be the different species observed, but it seems more probable to be a result of the paraffin technique. Schaxel (T9) mentions a swollen nerve ending with an apparently complex structure entering the hair lumen. None of these early studies describes an extracellular space beyond the hair base or the exact ending of the dendrite. Such details can hardly be seen in the light microscope and usually need confirmation from electron micrographs.
Tactile hairs have been studied more extensively in other arthropods, mainly in insects (Hsii, ’38; Schneider and Kaissling, ’57; Peters, ’62). The ultrastructure of tactile hairs is known from a grasshopper (Slifer, ’61), a blow-fly (Richter, ’64), the honey-bee (Thurm, ’64, ’65) and a beetle
106
RAINER F. FOELIX
(Moeck, ’68). Insect tactile hairs show close resemblance to the straight, sharp-pointed hair in Araneus. Thurm (’64, ’65) gave a fine structural and electrophysio-logical analysis of the hair plate sensilla in the honey-bee. The dendritic ending is enclosed in a scolopale and is attached eccentrically to the hair base; it contains a tubular body as well as electron dense granules of 200 Â diameter, but no other cell organelles. This corresponds to my observations in Araneus, £nd there are only few differences. (1) The dendritic ending is simply spindle-shaped in the honey-bee, but three-lobed in Araneus. It is not known whether the three-lobed structure bears any functional significance. Slifer (’61) and Richter (’64) found an irregular folding of the neural terminal just before attachment to the hair base. (2) The electron dense granules are much larger in Araneus (> 1500 Â diameter) and possess a more differentiated structure. Those granules were not reported by any of the other authors mentioned above.» 3) Two fiber strands of unknown material connect the dendrite laterally to the hair base. This may be similar to what Moeck (’68) describes in a tactile hair from the antenna of a beetle, where he found “cuticular strands’t connecting the scolopale to the hair base and a “tormogene vacuole” surrounding it. (4) The dendritic ending is also attached to the socket cuticle.
According to Thurm (’64) the extracellular space, which he calls “cap,” is filled by some “spongy, fibrous material”; compression of that cap should lead to excitation of the dendrite. In the straight, sharp-pointed hair of Araneus we find a fluid-filled cavity like in all the other tactile hairs of insects described so far (Slifer, ’61; Richter, ’64; Moeck, ’68). The direct contact of the dendrite on the one side and the surrounding extracellular fluid on the other side offer two possibilities for excitation. Either the stimulus is transmitted to the dendrite directly by pulling or pushing, or a compression of the fluid-filled cavity acts as a trigger for excitation. Thurm (’64 ) found in the tactile hair of the honey-bee that only compression of the cap is an effective stimulus, whereas pulling of the hair is not. If we replace the dense “cap” by the extracellular fluid, a
hydraulic compression could transmit the stimulus as is suggested for campaniform sensilla of insects by Chevalier (’69) or for lyriform organs of spiders by Salpeter and Walcott (’60). The narrow socket of the spider hair allows only a limited range of movement, maximally about 20°, whereas the tactile hair of the honey bee can be bent about 80°. It is questionable whether a minor displacement of the bristle can lead to an effective compression of the fluid-filled cavity. The attachment of the dendrite to the socket cuticle, which was not found in insect mechanosensitive hairs, certainly provides a possibility of direct deformation of the dendritic ending when the hair base is moved. The site of the tubular body is believed to be the receptive part of the dendrite, where the excitation originates (Thurm, ’64). This hypothesis matches well with the position of the tubular body in Araneus. For determining the actual stimulus mechanism, an electrophysiological approach is necessary.
In Araneus the neural terminal is always Btuated on the proximal side of the hair base, but in insect tactile hairs the dendrite can end either proximally, or eccentrically) (Peters,B’62; Thurm, ’64; Moeck, ’68) or in some cases centrally (Haskell, ’59) or it might enter the hair lumen for a short distance (Slifer, ’61; ’68)^The neural ending is never naked, but always enclosed in a scolopale. A protection of the nerve terminal seems to be a constant feature in most sensory cells (Pringle, ’62).
For several reasons it seems safe to conclude that the straight, sharp-pointed hairs as well as the serrated bristles are tactile in function. They are all innervated by one bipolar neuron, the dendrite of which is attached to the hair base. Any movement of the hair can therefore be transmitted to the dendrite. There is a great similarity between these hairs in Araneus and the mechanoreceptive hairs found in insects. The common hairs on the tarsus of the spider Cupiennus salei are phasic mechano-receptors as shown elec trophy siologic ally (Barth, personal communication). A possible chemoreceptive function can be excluded, as the cuticle wall of these hairs is rather thick and impermeable to dyes.
TARSAL HAIRS IN A WEB-BUILDING SPIDER
107
The serrated bristles have an additional function in grasping the thread, as proposed by Nielsen (’31). This could be proven directly through examination with the scanning electron microscope and indirectly by means of ablation experiments.
The claws, which have been studied only briefly, are supplied with several scolo-pales which enclose two or three dendrites each. The functional significance of the middle hook (grasping of the thread) is reflected in its large number of nerve processes; it contains five or six scolopales, whereas the main claws have but one or two. If these organs are actually mechano-sensitive, it would be a new example for a multiple-innervated mechanoreceptor. So far there is only one case known in spiders, namely the trichobothria, which have three or four dendrites connected to one sensil-lum (Gôrner, ’65; Christian, personal communication). The middle hook is present in all orb-web weavers but only in few hunting spiders (e.g., Lycosidae). The life in a vertical orb web apparently requires a specialized mechanical equipments such as serrated bristles and a movable hook. The interaction of both structures enables the spider to climb rapidly a single thread, to build a regular and efficient web or to hold on to the silk threads with only few legs, while wrapping and handling a prey.
ACKNOWLEDGMENTS
The author is indebted to Dr. P. N. Witt for advice and generous support, and to Dr. E. H. SJifer for valuable discussions. The expert assistance of Dr. I. R. Hagadorn and Dr. D. W. Misch in electron microscopy and of Mr. F. Anderson, Mr. B. Monk and Mr. G. Ross at the scanning electron microscope (Chemstrand Inc.) is gratefully acknowledged. This work was carried out in the laboratories of the Research Division of the North Carolina Department of Mental Health and was supported in part by NSF grant GB-6246X1 to Dr. P. N. Witt.
LITERATURE CITED
Andersen, S. O., and T. Weis-Fogh 1964 Resilin. A rubberlike protein in arthropod cuticle. Advances in Insect Physiol., 2: 1-65. Barth, F. G. 1967 Ein einzelnes Spaltsinnes-organ auf dem Spinnentarsus. Seine Erregung in Abhângigkeit von den Parametern des Luft-schallreizes. Z. vergl. Physiol., 55: 407-449.
– 1969 Die Feinstruktur des Spinneninte-
guments. I. Die Cuticula des Laufbeins adulter hâutungsferner Tiere (Cupiennus salei KEYS.). Z. Zellforsch., 97: 137-159.
Barth, F. G., and N. Deutschlânder 1969 Der Bau eines Einzelspaltsinnesorgans auf dem Tarsus der Spinne Cupiennus salei KEYS. Mem. Mus. nat. Hist, natur. (Paris), in press.
Bullock, T. HHand G. A. Horridge 1965 Structure and Function of the Nervous System of Invertebrates. Freeman, San Francisco and London.
Chevalier, R. L. 1969 The fine structure of campaniform sensilla on the halteres of Drosophila melanogaster. J. Morph., 128: 443-464.
Comstock, J. H. 1948 The Spider Book. Corn-stock Publ. Comp., Ithaca, N. Y.
Frank, H. 1957 Untersuchungen zur funk-tionellen Anatomie der lokomotorischen Ex-tremitâten von Zygiella x^notata, einer Rad-netzspinne. Zool. Jahrb. Abt. Anat., 76: 423— 460.
Gôrner, P. 1965 A proposed transducing mechanism for a multiply-innervated mechanoreceptor ( trichobothrium ) in spiders. Cold Spr. Harb. Symp. quant. Biol., 30: 69—73.
Gôrner, P., and P. Andrews 1969 Trichoboth-rien, ein Ferntastsinnesorgan bei Webespinnen
:0%^Araneen). Z. vergl. Physiol., 64: 301^317.
Gossel, P. 1935 Beitrâge zur Kenntnis der Hautsfnnesorgane und Hautdrüsen der Cheli-ceraten. Z. Morph. Ôkol. Tiere, 30; 117-205.
Haskell, P. T. 1959 Function of certain pro-thoracic hair receptors in the desert locust. Nature (Lond.), 183: 1107.
Hsü, F. 1938 Etude cytologique et comparée sur les sensilla des Insectes. Cellule, 47: 5-61.
Mclndoo, N. E. 1911 The lyriform organs and tactile hairs of araneads. Proc. Acad. Nat. Sci. Philad., 63: 375-418.
Millonig, G. 1961 Advantages of a phosphate buffer for Os04 solutions in fixation. J. Appl. Phys., 32: 1637.
Moeck, H. A. 1968 Electron microscopie studies of antennal sensilla in the ambrosia beetle Trypodendron lineatum (Oliver) (Scolytidae), Can. J. Zool., 46: 521-575.
Nicklaus, R., P. G. Lundquist and J. Wersàll 1967 Elektronenmikroskopie am sensorischen Apparat der Fadenhaare auf den Cerci der Schabe Periplaneta americana. Z. vergl. Physiol., 56; 412-415.
Nielsen, E. 1931 The Biology of Spiders. Vol. I. Levin & Munksgaard, Copenhagen.
Peters, W. 1962 Die proprioceptiven Organe am Prosternum und an den Labellen von Calliphora erythrocephala Mg. (Diptera). Z. Morph. Ôkol. Tiere, 51: 211-226.
Pringle, J. W. S. 1962 Prologue: the input element. Symp. Soc. exp. Biol., 16, Biol. Rec. Mech. Univ. Press, Cambridge.
Reed, C. F. 1969 Cues in web building. Am. Zoologist, 9: 211-221.
Reed, C. Fij P. N. Witt and R. L. Jones 1965 The measuring function of the first legs in Araneus diadematus Cl. Behaviour, 25: 98-119.
Richardson, K. C., L. J. Jarret and E. H. Finke 1960 Embedding in epoxy resins for ultrathin
108
RAINER F. FOELIX
section in electron microscopy. Stain Technol., 35: 313-323.
Richter, S. 1964 Die Feinstruktur des für die Mechanorezeption wichtigen Bereichs der Stel-lungshaare auf dem Prosternum von Calliphora erythrocephala Mg. (Diptera). Z. Morph, ôkol. Tiere, 54: 202-218.
Sabatini, D. D., F. Bensch and R. J. Barrett 1963 Cytochemistry and electron microscopy. The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J. Cell Biol., 17: 19-58.
Salpeter, M. C., and C. Walcott 1960 An electron microscopical study of a vibration receptor in the spider. Exptl. Neurol., 2: 232-250.
Schaxel, J. 1919 Die Tastsinnesorgane der Spinnen. Jena Z. Naturwiss., 56: 13-20.
Schneider, D., and K. E. Kaissling 1957 Der Bau der Antenne des Seidenspinners Bombyx mori L. II. Sensillen, cuticulare Bildungen und innerer Bau. Zool. Jahrb. Abt. Anat. Ontog. Tiere, 76: 223-250.
Slifer, E. H. 1961 The fine structure of insect sense organs. Intern. Rev. Cytol., 11: 125-159.
– 1968 Sense organs on the antennal
flagellum of a giant cockroach Gromphadorina portentosa, and a comparison with those of several other species (Dictyoptera, Blattaria). J. Morph., 126: 19-30.
Thurm, U. 1964 Mechanoreceptors in the cuticle of the honey bee; fine structure and stimulus mechanism. Science, 145: 1063-1065.
– 1965 An insect mechanoreceptor. Part
I. Fine structure and adequate stimuli. Cold Spr. Harb. Symp. quant. Biol., 30: 75-82.
Venable, J. H., and R. E. Coggeshall 1965 A simplified lead citrate stain for electron microscopy. J. Cell Biol., 25: 407-408.
Weis-Fogh, T. 1960 A rubber-like protein in insect cuticle. J. Exp. Biol., 37: 889-907.
Wilson, R. S. 1962 The control of dragline spinning in the garden spider. Quart. J. Micr. Sci., 104: 557-571.
Witt, P. N. 1956 Die Wirkung von Substanzen auf den Netzbau der Spinne als biologischer Test. Springer, Berlin-Gottingen-Heidelberg.
Witt, P. N., C. F. Reed and D. B. Peakall 1968 A Spider’s Web. Springer, Berlin-Heidelberg-New York.
PLATE 1
EXPLANATION OF FIGURES
1 Photograph of the tip of a tarsus of a typical orb-web weaver (Neoscona sp.) showing two main claws (M), one out of focus, one middle-hook (m), several serrated bristles (s), rows of dark, sharp-pointed hairs (t) and rows of light, blunt-tipped hairs (c). Note the single slit sensillum just proximal to the claws (arrow), x 225.
2 The three types of bristles on the tarsus of Araneus diadematus: straight, sharp-pointed hair (t), curved, blunt-tipped hair (c) and serrated bristles (s). Insertion of the main claw is seen in the background. For gross morphology of these bristle types compare with figure 1. X 980.
PLATE 1
TARSAL HAIRS IN A WEB-BUILDING SPIDER Rainer F. Foelix

109

PLATE 2
EXPLANATION OF FIGURES
3 Scanning electron micrograph (SEM) showing that the straight, sharp-pointed hair arises at an angle of about 30° to the leg axis, whereas the curved, blunt-tipped hairs (c) form an angle of 50° to 75°. Note the scaly appearance of the leg cuticle, the smooth surface of the sockets and the various striae on different portions of the straight hair. X-1400.
4 The surface structure of a single straight, sharp-pointed hair. Longitudinal striae fuse towards the tip and therefore diminish in number. Each stria is composed of fine ribs, arranged in a V-pattern. Small projections cover the surface irregularly. (SEM) x 2600.
5 Surface structure close to the tip, at a higher magnification. Note that the spiraling striae end at a dorsal ridge. (SEM) X 7500.
110
TARSAL HAIRS IN A WEB-BUILDING SPIDER Rainer F. Foelix
PLATE 2



PLATE 3
EXPLANATION OF FIGURES

7 Diagram of the innervation of a straight, sharp-pointed hair.J|a) A longitudinal section at the level of the light microscope shows the hair (h) being suspended obliquely in a slipper-shaped socket (so) in the cuticle (c). A bipolar neuron (nr), embedded in the hypodermis (Hy), sends a long dendrite (d) close to the hair base. The three-lobed dendritic ending is surrounded by a fluid-filled extracellular space (es); no direct contact to the hair base is visible. The hypodermis cell (tormogene cell ?) underlying the extracellular space contains a large amount of crystalline deposits (cry), (b) Details of the hair innervation as seen with the electron microscope. The hair base (hb) is attached to the socket cuticle (sc) by means of a fibrous connecting membrane (cm). The dendritic ending (d) makes contact with the hair base in two different ways: (1) directly by a fine extension of the middle lobe and (2) indirectly by intercalated cuticular (?) fibers (f). The three-lobed dendritic ending contains a tubular body and a peripheral layer of granules of various densitySits cuticular sheath seems to be fastened to the socket cuticle. At least one sheath cell (S) encloses the dendrite proximally. Numerous microvilli (mv) face the extracellular cavity. N, nucleus; P, pigment granules of hypodermis cells; hi, hair lumen.
6 Longitudinal section of the base of a straight, sharp-pointed hair. The dendrite of a bipolar neuron (arrow) ends in a three-lobed structure proximal to the hair base (h) just below the socket cuticle (sc). Between the hair base and the hypodermis (hy) lies a fluid-filled extracellular space (es). The cell underlying the hair base contains numerous crystalline deposits (cry; compare with fig. 7), hi, hemolymph.H||L250.
8 Innervation of the hair (h) as seen in the electron microscope (longitudinal section). The dendritic ending is enclosed in a scolopale (s); it contains a tubular body (Tb) in the center and a layer of granules at the periphery. Some extracellular fibers (f ) surround the scolopale and connect the dendritic ending to the hair base. Another portion of the dendrite seems to be fixed to the cuticle of the socket (arrows). Numerous microvilli (mv) face the extracellular space. Note the almost structureless appearance of the connecting membrane (cm). X 13,400.
9 Electron micrograph of a longitudinal section of the three-lobed dendritic ending surrounded by formative cells (fresh-molted animal). The middle lobe extends into a fine channel, which makes contact with the hair base (h).^^18,500.
10 Tangential section of a straight, sharp-pointed hair. Note the bulbous ending of the dendrite (d) and the two strands, each connecting to one side of the hair base (arrows). X 1450.
11 Para-median section of dendritic ending. Note the varying density in the granules and the thick membrane bounding them (arrows). The tubular body (Tb) shows a very fine cross-striation, being spaced at 250 Â. Note also the close relation between the scolopale (s) and the socket cuticle (sc). X 40,000.
TARSAL HAIRS IN A WEB-BUILDING SPIDER Rainer F. Foelix
PLATE 3
113





114
PLATE 4
EXPLANATION OF FIGURES
12 Ventral view of the serrated bristles and middle hook (m) on leg 4 with the SEM. Note the bulbous base, teeth and longitudinal surface striation of each bristle. In the lower left corner a single slit sensil-lum is shown (arrow).® 1400.
13 Front view of the tip of the fourth leg as seen with the SEM (leg pointing out of the plane of the paper). The strong, three-toothed bristle stands on the innerside towards the abdomen, whereas the middle hook points towards the o®side. The spine beyond the serrated bristles is called “sustentaculum” MS) and occurs only on leg 4.
ÿ: 800.
14 Mechanism of grasping the thread with leg 1. The silk thread is pushed into the little notches of the serrated bristles by means of the middle hook. The dotted circles indicate the insertion of curved, blunt-tipped hairs. Two slit sensilla border the serrated bristles proximally.

TARSAL HAIRS IN Rainer F. Foelix
A Web-building
SPIDER

PLATE 4

PLATE 5
EXPLANATION OF FIGURES
15 Leg 1 hooked on a thread |||Tj) of the Jhib region. Only the middle hook is involved in grasping the thread, whereas the main claws are free. (SEM ||M 880.
16 Mechanism of grasping the thread: the thread (dotted, so it is recognizable ) is pushed into the little notches^Hthe sÆpted bristles (arrow) and held by the Biddle hook (leg 1 jBplote also three blunt-tipped hairs pointing ventrallS ( SEM ). B 1700.
116
TARSAL HAIRS IN A WEB-BUILDING SPIDER Rainer F. Foelix
PLATE 5

117

PLATE 6
EXPLANATION OF FIGURES
17 Leg 3 after removal of all serrated bristles. The arrow indicates a single slit sensillum (SEM). x 200.
18 A representative control web of a female Araneus diadematus before removal of all serrated bristles. Scale in upper left corner: 1 Division, 2 cm.
19 The sixth web built by the same spider after removal of all serrated bristles on all legs showing the typical irregular web pattern.
20 The second web built by the same spider after molting, when all serrated bristles had regenerated. The regularity of the control web is almost re-established.
118
TARSAL HAIRS IN A WEB-BUILDING SPIDER Rainer F. Foelix
PLATE 6




PLATE 7
EXPLANATION OF FIGURES
§1 Cross-section of a tarsus of leg 1, just behind the claws. Main claws CM.) are supplied by two nerve fibers, middlehook (m) by six fibers, enclosed in a cuticular sheath. Note also cross-sections of straight, sharp-pointed hairs (t>, curved, blunt-tipped hairs (c) with double lumen, and irregular shaped, serrated bristles (s). (figs. 21, 22 and 23 show sections from freshly molted animals )n475.
22 Cross-section of a scolopale running into a main claw, which contains two or three dendrites. The ten double tubules (small arrow) seen in one of the dendrites suggest a ciliary nature. The cells surrounding the scolopale, probably glial cells, contain many microtubules, some mitochondria (m) and desmosomal cell junctions (arrow).
^^37,000.
23 Ending of a scolopale inside the cuticle of a main claw. Note the numerous microtubules and the dense material surrounding the dendrite, m 33,000.
120
TARSAL HAIRS IN A WEB-BUILDING SPIDER Rainer F. Foelix
PLATE 7



PLATE 8
EXPLANATION OF FIGURE
Metatarsal lyriform organ close to the joint (j) of tarsus and metatarsus on leg 4. The tarsus itself bears only single slit sensilla but no lyriform organs. (SEM).jlJ 2000.
TARSAL HAIRS IN A WEB-BUILDING SPIDER Rainer F. Foelix
PLATE 8

123
/
4