Images Collection
View this article in Search Friendly Plain Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
Am. Zoologist, 9:71-79 (1969).
Synthesis of Silk, Mechanism and Location David B. Peakall
Department of Pharmacology, Upstate Medical Center, Syracuse, New York
Synopsis. The location and function of the five or six sets of silk glands of Araneus diadematus (Cl) are discussed. The structure and function of the three major parts of the ampullate gland indicate a synthesizing, collecting, and possibly structuring section. Two methods of stimulation of the ampullate gland, namely emptying the gland and cholinergic stimulation, are known. In both cases there is an initial secretory stage followed by rapid synthesis of new protein. The sequence of events following stimulation by both methods is described, based on studies of the incorporation of labeled protein and RNA precursors and on autoradiographic studies. Characteristic changes occur in the fine structure during the stimulatory cycle. Several experiments show that the spider has information on the amount of silk available to it for use in web-building. A structure which may act as a biological transducer has been located in the ampullate gland.
Spiders have evolved to make the maximum use of that remarkable protein, silk. To judge from the wide geqgraphic distribution, the number of species as compared to the other orders of the Class Arachnida, and the numbers of individuals per unit area of some species, this exploitation of silk has been successful. Although the spider has successfully made use of silk, it has in so doing placed itself in the position described rather picturesquely by the French zoologists, André and Lamy, “Spiders lead lives bristling with difficulties and are always on the horns of a dreadful dilemma: no food without webs and no webs without food.”
To’minimize the dangers of running out of silk, the silk gland is prepared for immediate action. Not only is the cytoplasm full of droplets of silk ready to be secreted into the lumen, but the .major amino acids of which the silk is comprised are present in high concentrations ready for new synthesis to start. Spiders economize on usage of silk as far as possible by redigesting every scrap of it. The provi-
Present address: Division of Ecology and Sys-tematics, Langmuir Laboratory, Cornell University, Ithaca, N. Y. 14850
Supported by research grants PN-5234 from the National Institutes of Health and GB-5736 from the National Science Foundation. Portions of these studies were carried &ut while the author was an Established InvestigaéH^ American Heart Association.
sional spiral of the web, for example, is eaten as the final sticky spiral is laid down. Examination of serial sections made of the spider just after its web is completed shows that those glands which produce silk for the web are largely empty. The spider’s operating margin is small.
The ampullate glands of Araneus serica-tus comprise 3-5% of the body weight of the spider on a wet-weight basis, and are able to produce protein equivalent to 10% of the gland’s weight every web-building cycle. It is this frequent, rapid production of a single structural protein that makes these glands an attractive model for the study of the regulation of protein synthesis. Another useful feature is that the amino acid composition is unusual, the two amino acids, alanine and glycine, comprising some 57% of the protein. Thus, it is possible to follow changes in the amino acid pools that would be difficult if the composition of the protein were more normal.
Although studies of the structure and function of the silk glands of the spider date back to Blackwell’s observations in 1839, there is still no final agreement on the number of sets of silk glands. The types reported for Araneus diadematus and Araneus sericatus, together with their number, location, and function are listed in Table 1. The larger gl^|k shown dissected out of an adult female sericatus
71
72
David B. Peakall
table 1. Number and ‘position of each type of silk gland and the function of the silk. The number of glands is based largely on the work of Warburton (1890), the modification below the line on the studies of Sekiguchi (1952). The function of the silk is based on Apstem (1889), Warburton (1890), Comstock (1948), Sekiguchi (1952), md Peakall (1964).
Classification of spinnerets
| Type of silk gland | Anterior | Median | Posterior | Function of silk |
| Ampullate | 2 | 2 | — | Drag-line, scaffolding of web, base-line of catching spiral? |
| Aggregate | — | — | 6 | Viscid thread of catching spiral |
| Cylindrical or tubulif orm | — | 2 | 4 | Egg sac |
| Piriform | 200 | — | — | Attachment discs |
| Aciniform | — | 200 | 200 | Swathing bands |
| Aggregate | — | •— | 4 | Viscid thread of catching spiral |
| Flagelliform* | — | — | 2 | Base-line of catching spiral |
* Named coronata independently by Peters (1955).
in Figure 1. Personally, I cannot locate the flagelliform glands either histologically or by dissection. The studies on the synthesis of silk have been largely confined to the ampullate glands, and it is these glands that are considered unless otherwise stated. Limited observations suggest that the mode of stimulation of the aggregate gland is similar to that of the ampullate gland, whereas that of the cylindrical gland is quite different (Peakall, 1965).
The ampullate gland consists of three morphologically distinct parts, the tail, the sac, and the duct (Fig. 1). The main, probably the sole, function of the long tail of the gland is the synthesis of silk protein. The fine structure of, and the biochemical changes in, these cells during the protein-synthesizing cycle will be discussed later.
An important function of the sac of the gland is the storage of protein (Fig. 1). The volume of the lumen of the sac is twice that of the tail. The rate of synthesis of protein in the epithelial cells of the sac is only a quarter of that found in the tail. Since the volume of the epithelial cells of the tail is ten times that of the sac, the amount of protein synthesized in the sac is only a few percent of the total protein synthesized by the gland. However, electron microscopy indicates that the function of the epithelial cells of the sac may be complex. Five types of droplets have been found (Bell and Peakall, 1969). One of these appears to be identical with the silk protein droplets fojund in the tail, and another is similar to the material secreted
in the transitional zone between the sac and the duct. A third type is similar to lipid, and this can be correlated with increased lipid staining observed under the light microscope in the region of the sac as compared to the tail. The function of the other two types of droplets is unknown.
In the transitional zone between the duct and sac there is a region where the epithelium is composed of secretory cells. Electron micrography shows that these

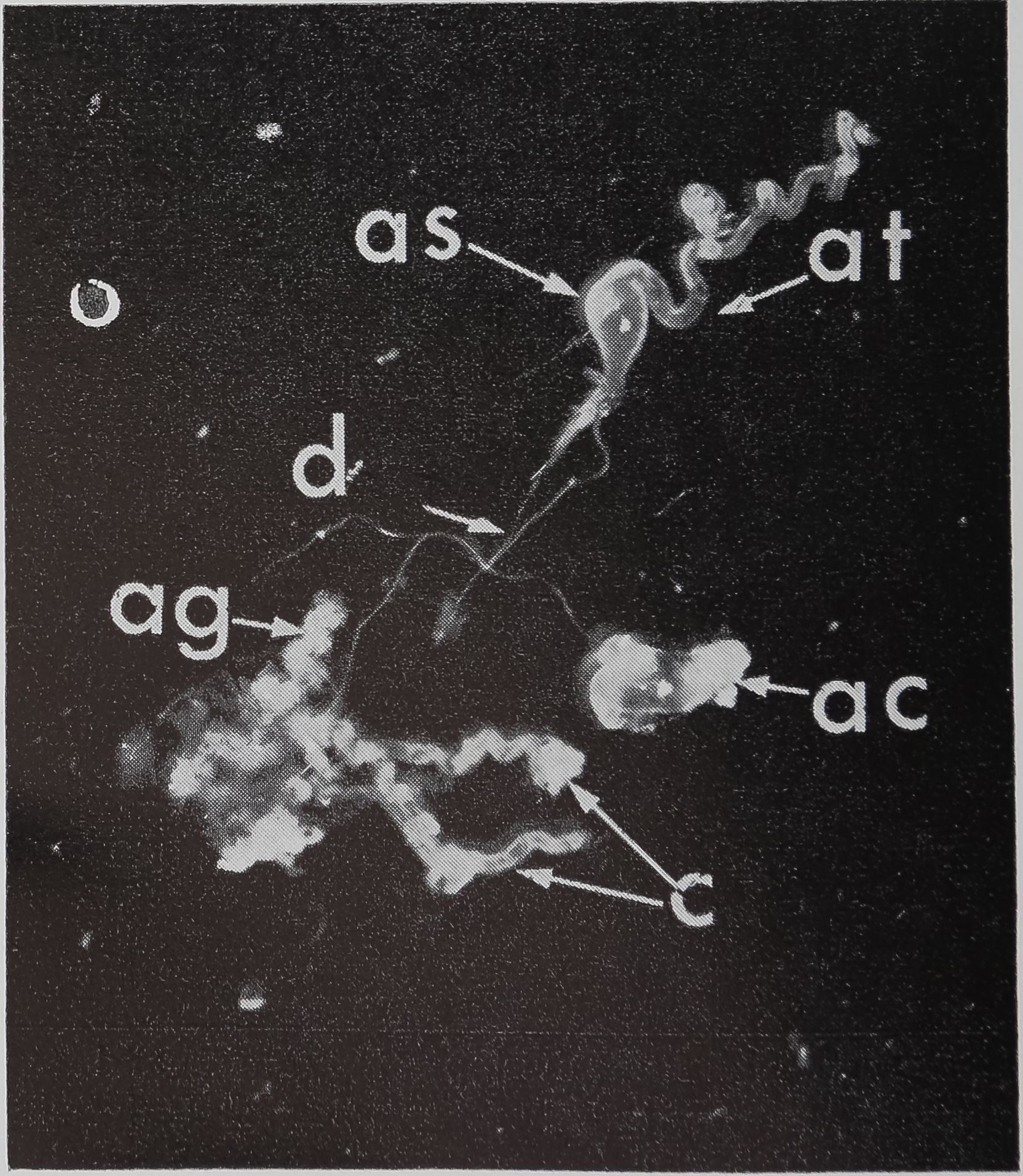
FIG. 1. The aggregate glands (ag), cylindrical glands (c), and two ampullate glands dissected out of an adult female Araneus sericatus. One ampullate gland is coiled (ac), the other is straightened out to shèw thfe various parts: at, tail; as, sac; d, duct. No staining, x 5
Synthesis of Silk
73
table 2. Two wa/ys of stimulating synthesis, of sillc in ampullate glcmds.
la. Acetylcholine binds with receptor on outer (basal) membrane of gland
lb. Emptying of the gland
2. Signal after release of presynthesized fibroin from epithelium
Signal across outer epithelial membrane causes release of presynthesized fibroin
Signal across inner (luminal) epithelial membrane causes release of presynthesized fibroin
Increase in nuclear size ; change in concentration of ENA ; increased incorporation of amino acids
Atropine-sensitive
Puromycin-insensitive
Atropine-insensitive
Puromycin-insensitive
Puromycin-sensitive
droplets have an electron density different from silk. One possible explanation of these droplets would be that they are a polymerase. It has been shown for the orb-web builder, Nephila madagascariensis, that there is a change of molecular weight from 30,000 in the sac to 200-300,000 in the completed fiber (Braunitzer and Wolff, 1955).
The duct is some five times longer than would be necessary if it had the sole function of connecting the main part of the gland to the spinnerets. The electron microscope reveals that the wall of the duct consists of microvilli arranged radially to the lumen of the duct (Bell and Peakall, 1969). It seems likely, on morphological grounds, that one function of the duct is absorption of water. Preliminary experiments have shown that the water content of the silk in the sac is considerably higher than that in the final fiber.
The long duct may also be of importance in the preliminary stages of orientation of the silk molecules. It is known that the silk in the sac is an «-configuration, whereas in the fiber it is in the form of a ^-pleated sheet (Ambrose, et al., 1961; Lenormant, 1956; Warwicker, 1960). However, Wilson, (1962, and this symposium) considers that the main orientation of the fiber occurs between the control valves at the base of the spinneret and the spigot. A further possibility is that it is necessary to have enough material in the duct to make a single radius. It has been noted that the time spent in the center of the web is long compared with that taken to lay down the radii (Witt, et al., 1968). It is possible that the pauses between construction of radii may be necessitated by the need to
pump silk into the duct. These various possibilities are not mutually exclusive.
Two ways of stimulating the ampullate gland to produce protein have been found (Peakall, 1966). These are emptying the silk from the gland and administering cholinergic agents. The first mode of stimulation is insensitive to atropine, whereas the second mode is blocked by this anticholinergic agent. In both cases the first stage of the cycle of stimulation is the secretion of preformed protein droplets from the epithelium into the lumen. This is followed by accelerated synthesis of new protein. The two steps, secretion and synthesis, can be separated by pretreatment with a blocker of protein synthesis, such as puromycin or actinomycin D. In this case the stimulation causes the secretion of preformed protein, but this is not followed by the synthesis of new protein. The modes of stimulation and the two-stage cycle of events following stimulation are listed in Table 2.
The sequence of events following stimulation of the ampullate gland will now be considered in more detail. Autoradiographic evidence (Peakall, 1958) shows that acetylcholine binds to the cell membrane but does not enter the cell. The first histological change observed is that the protein stored in the cytoplasm moves towards the luminal end of the cell and is discharged into the lumen. This is accompanied by fusing of droplets of protein; often the shape of the nucleus is distorted by this action (Fig. 2a, b). This change is in full swing within ten minutes of stimulation, and experiments with actinomycin D, which blocks the DNA-dependent synthesis of RNA, show that the signal for increas-
74
David B. Peakall



d. 60 min after stimulation.
ing the rate of synthesis occurs within this time. The amount of incorporation of C14-alanine in an hour following stimulation with acetylcholine was measured auto-radiographically. The results clearly show that the synthesis of protein is inhibited only if actinomycin D is given within three minutes of stimulation (Table 3). After this time, presumably enough messenger RNA is present to continue with the synthesis of new protein. The half-life of messenger RNA has been found to be approximately four hours. Experiments were carried out in which new synthesis of messenger RNA was blocked 15 minutes after stimulation, and the amount of incorporation of C14-alanine was measured at various time intervals and compared to controls (Table 4).
The movement of ribosomes during the stimulatory cycle has been examined in the light and electron microscopes, and by
table 3. Autoradiographic counts to show the degree of incorporation of Cu-alanine following stimulation with acetylcholine and subsequent administration of actinomycin D. Acetylcholine (1 mg/Teg) and Cu-alanine (5 ^c) were injected into the body fluids at the start of the experiment. Actinomycin D (0.1 pg/Tcg) was injected at stated intervals after the injection of acetylcholine.
Interval between Ach and actinomycin D injections (minutes)
Autoradiographic counts per mm* 1 2 3 4 5 (X 1940). Average of 100 counts with standard deviation; number of spiders used in parentheses.
0
1
2
3
4
5 10
Control (no actinomycin)
2.0 ± 1.7 (2) 1.4 ± 0.9 (2) 3.3 ± 1.3 (2) 3.9 ± 1.6 (2) 26.9 ± 5.7 (2) 25.3 ± 6.0 (3) 33.7 ± 5.5 (2) 31.6 ± 6.3 (4)
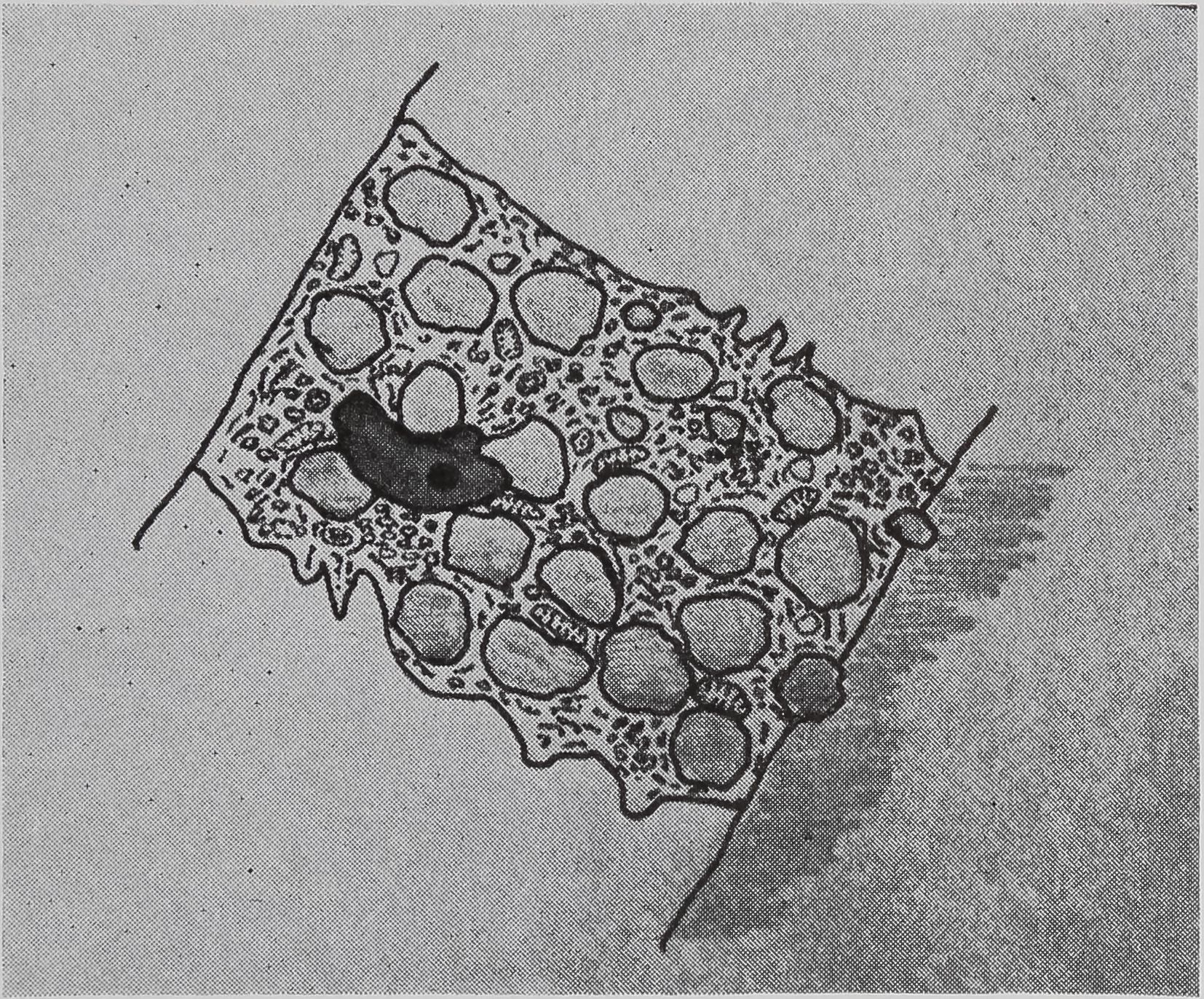
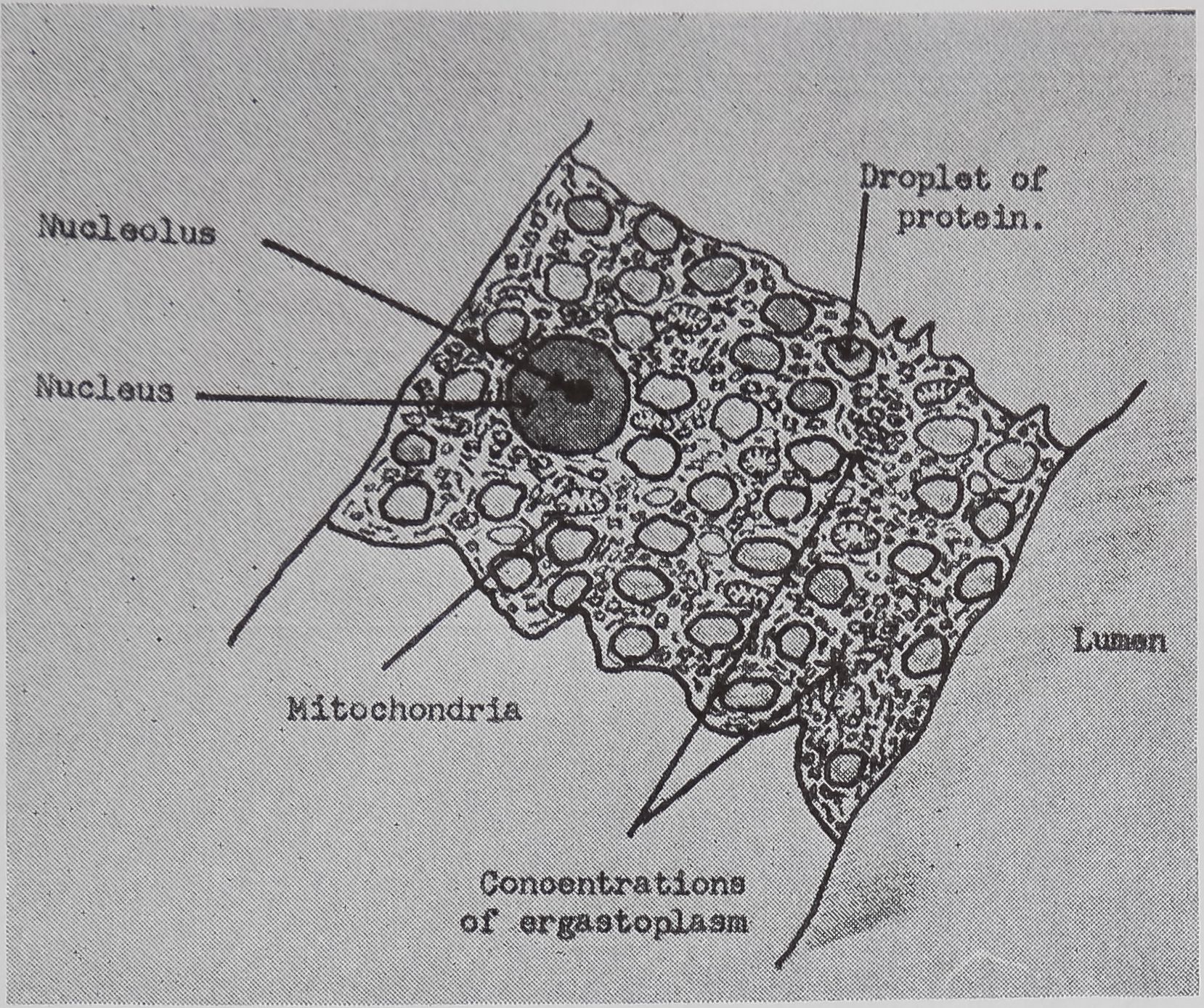
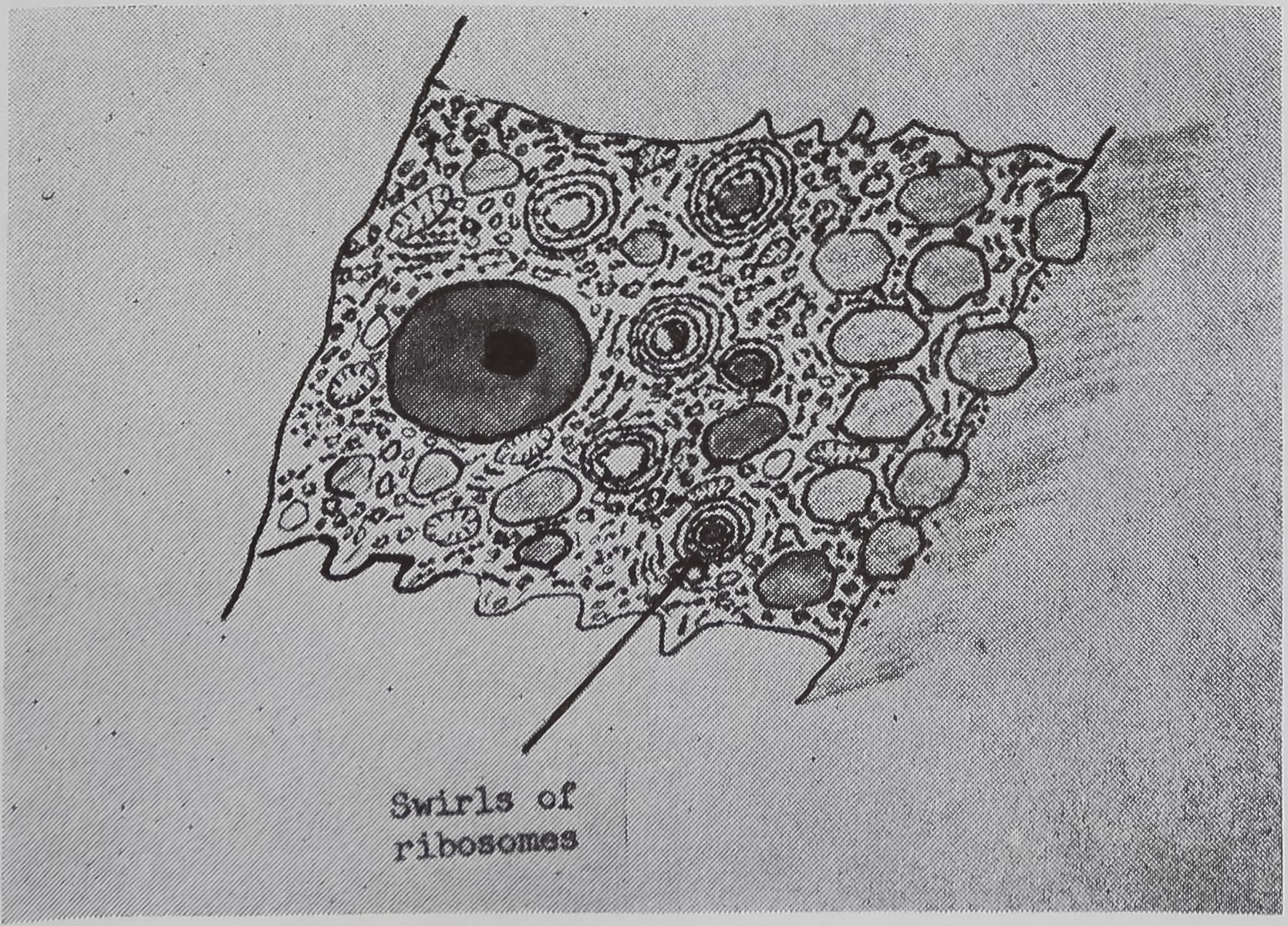
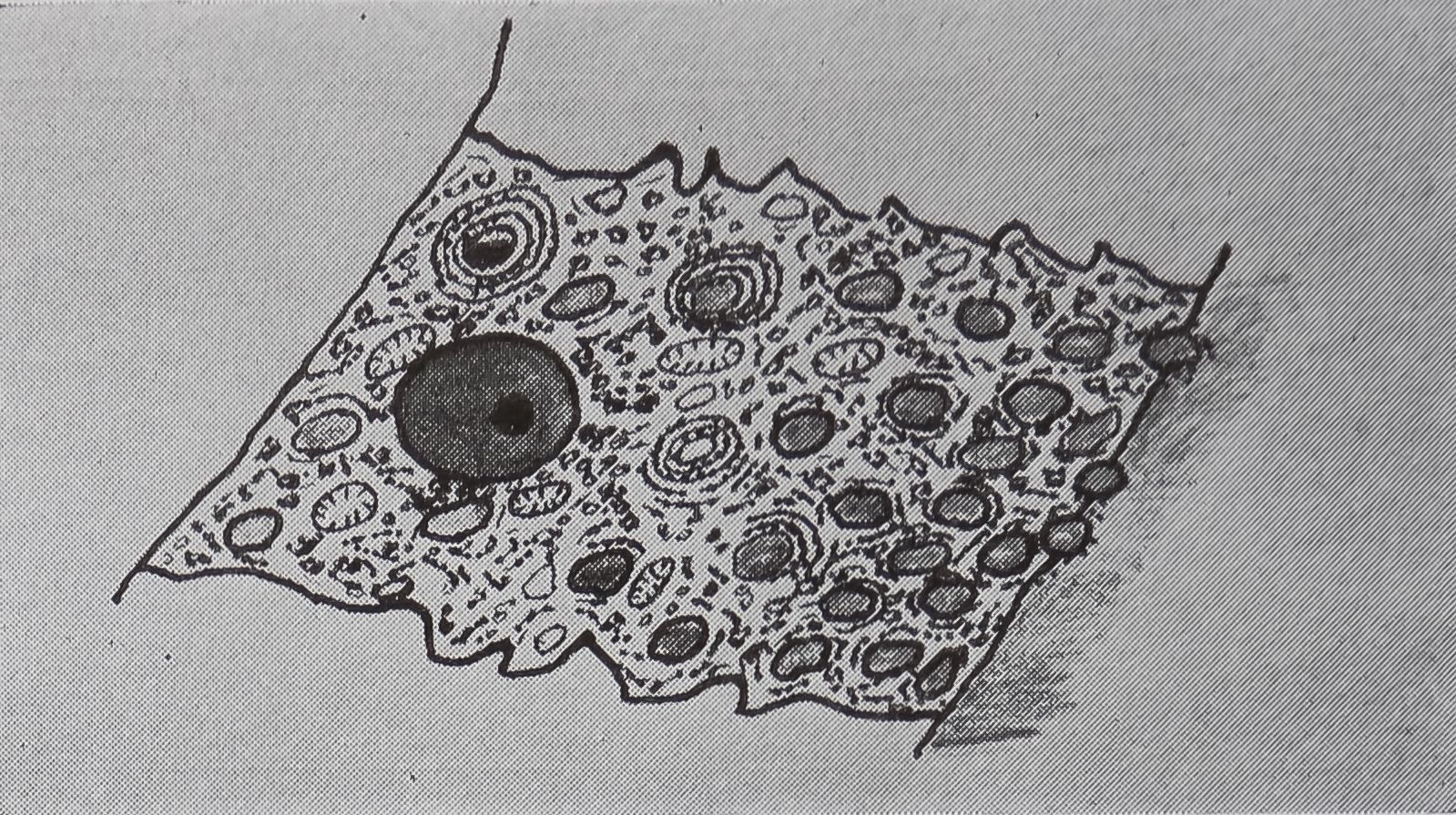
FIG. 2. Diagrammatic representation of the structural changes occurring in the epithelial cells of the tail of the ampullate gland with stimulation. Based on light and electron microscopic studies, a. Appearance of cell before stimulation.
b. 5-10 min after stimulation.
c. 20 min after stimulation.

Synthesis of Silk
75
table 4. All spiders stimulated by injecting acetylcholine (1 mg/leg) into the body fluids. Experimental animals given actinomycin D (1 y/leg) by the same route 15 mimtes later* Cn-Alanine was given at various intervals after the blocicing of ENA synthesis by aetinomydii. The amount of incorporation in 1-hr periods was measured at various times and expressed as cpm/y ENA.
Each figure is the average, with standard deviation, of four spiders.
| Incorporation cpm/7 RNA | ||||
| 1-2 hrs | 2-3 hrs | 4-5 hrs | 6-7 hrs | |
| Experimental | 256 ■+■ 35.5 | 147 -+- 23.9 | 92 *+■ 17.8 | 23 H- 4.5 |
| Control | 349 ± 41.7 | 248 ± 31.0 | 189 ± 25.3 | 106 ± 16.4 |
| Experimental % of control | 73 | 59 | 49 | 22 |
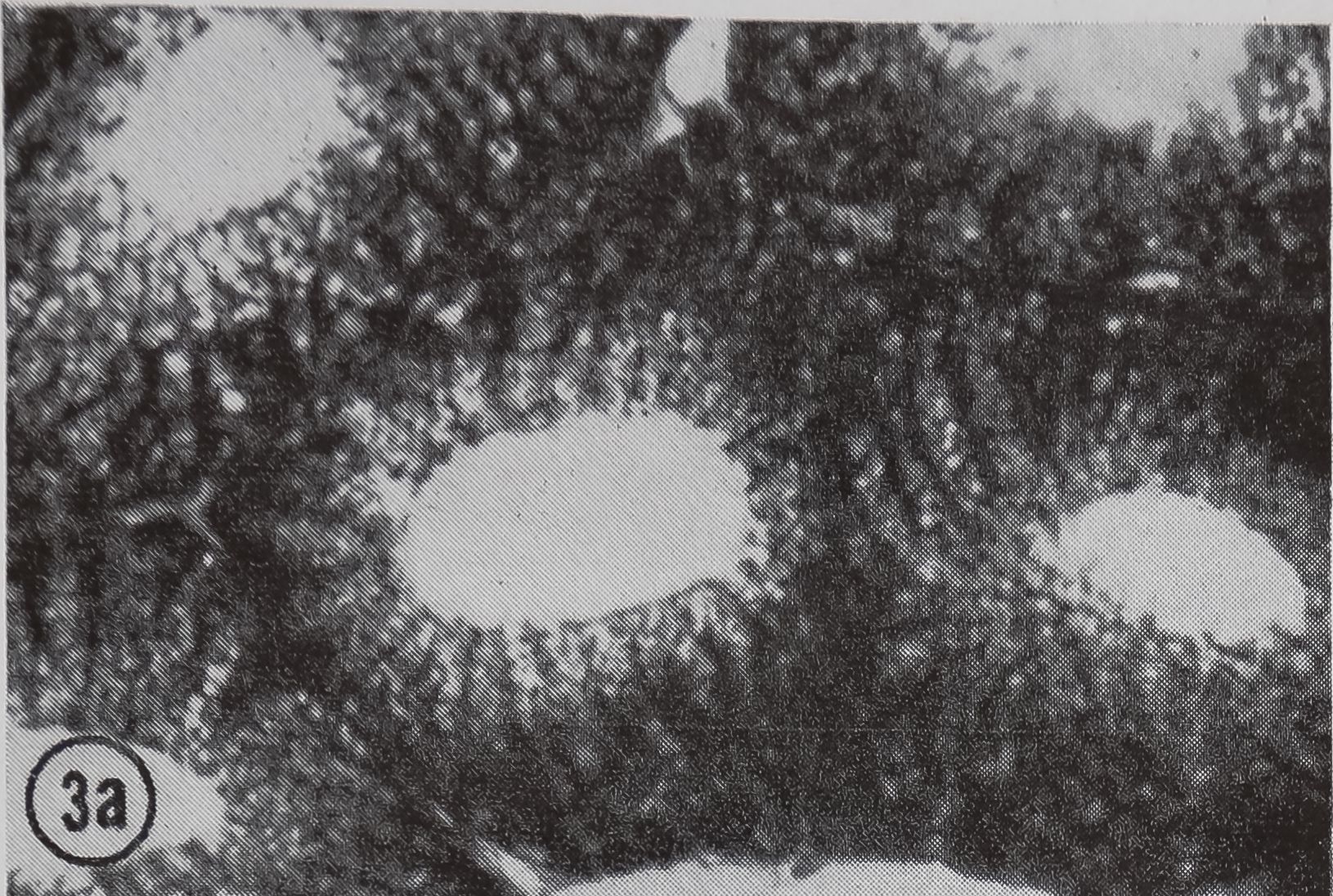
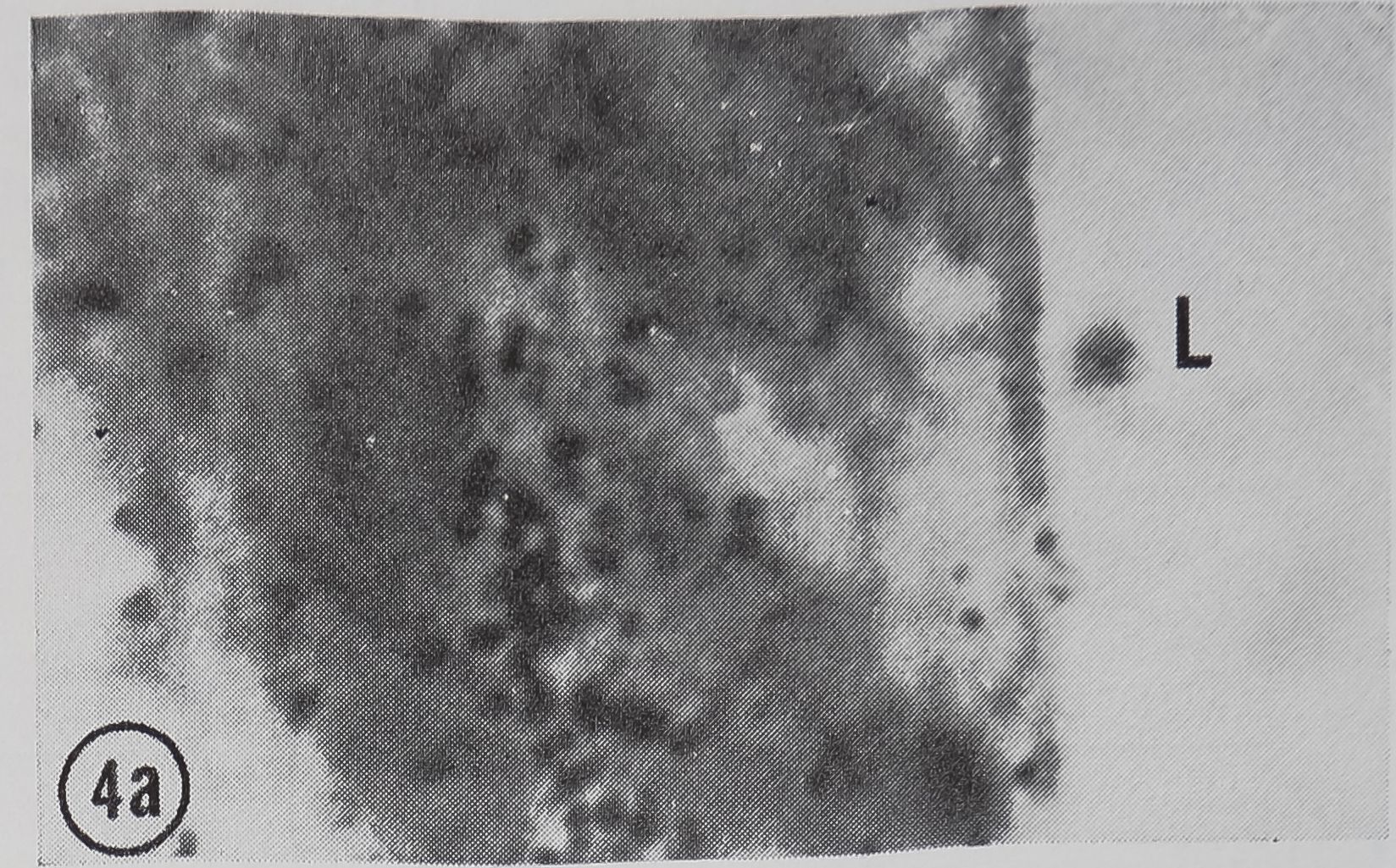
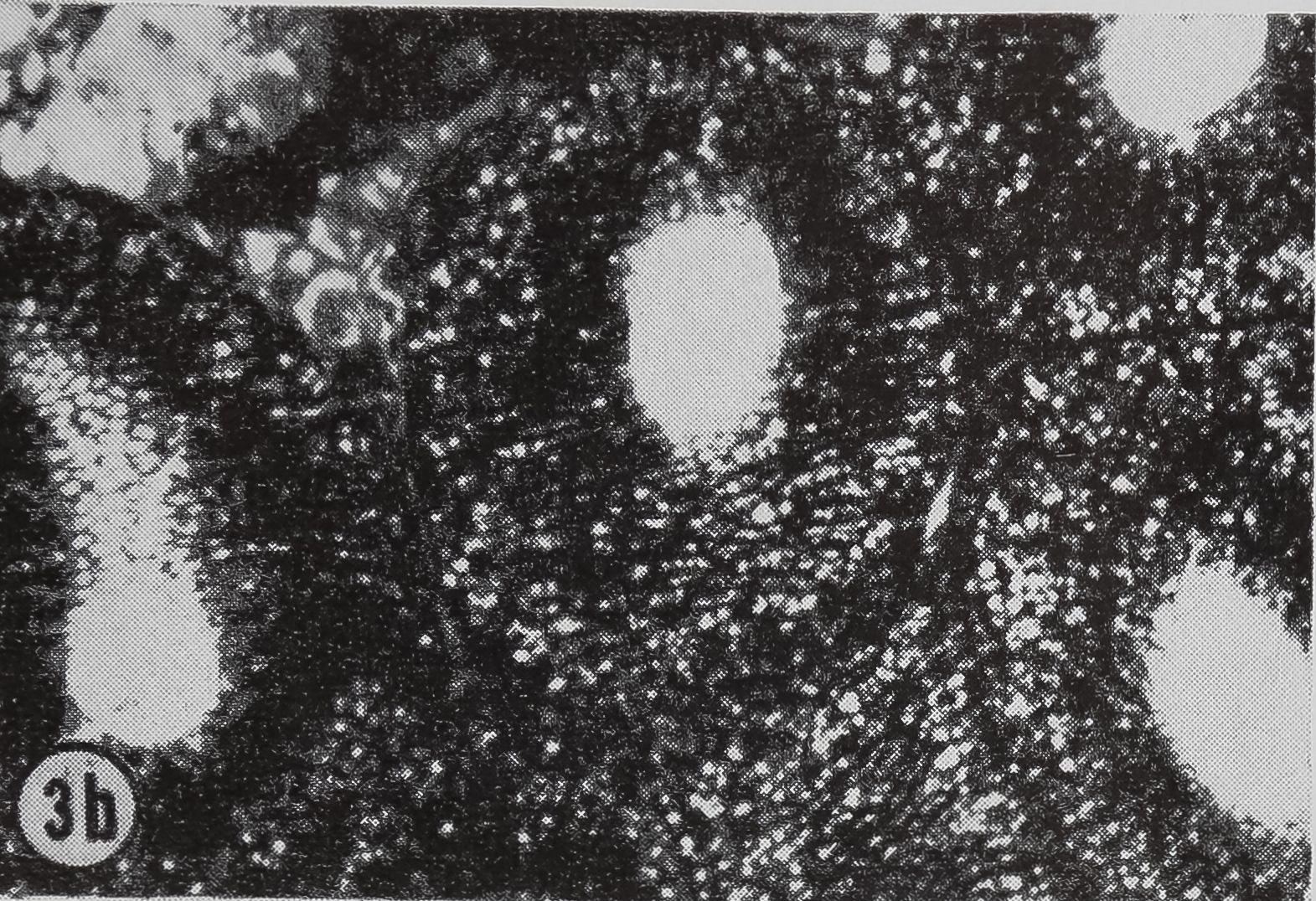
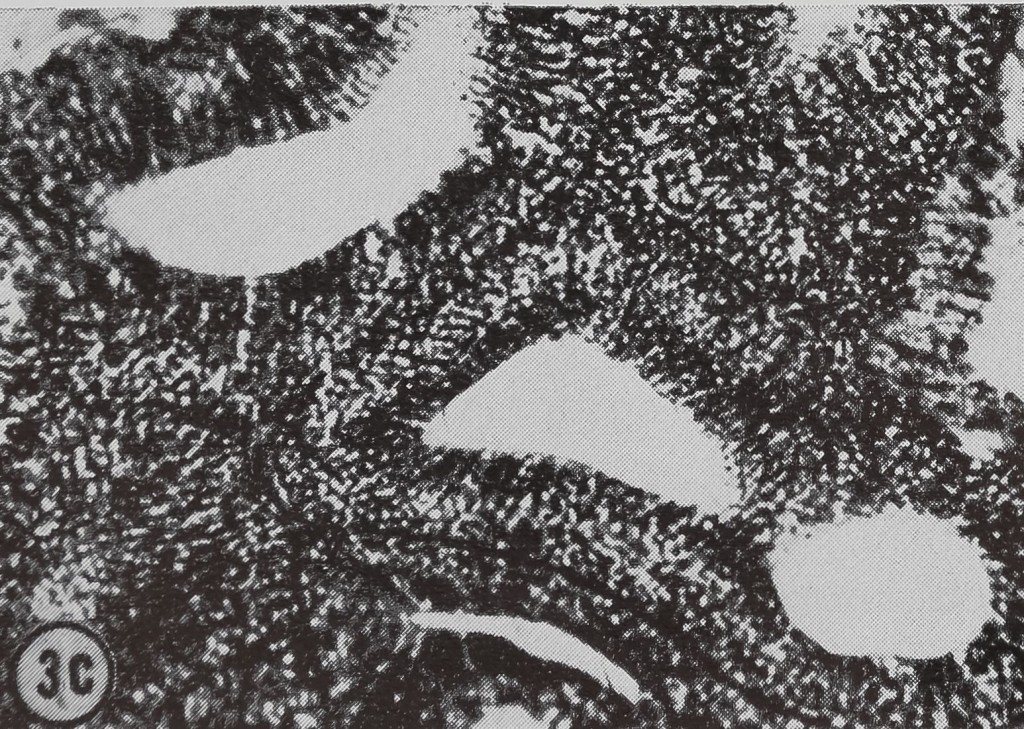
following a pulse of labeled orotic acid with light autoradiography. Ten minutes after stimulation, the RNA is concentrated in the basal half of the cell (Figs. 3a and 4a). Twenty minutes after stimulation it is more generally distributed but with some concentration in the luminal half (Fig. 3b), which is particularly clear in the autoradiograph (Fig. 4b). After an hour there is little localization of RNA (Figs. 3c and 4c). Electron microscopy shows that new protein droplets are associated with characteristic whorls of ergastoplasm (Fig. 2c). It is of interest that no Golgi apparatus is found in the tail of the gland. It is possible that the by-passing of this packaging step is an adaptation for allowing rapid synthesis.
The course of protein synthesis has been followed by giving a short pulse of labeled alanine at the time of stimulation. It is found that active synthesis and secretion into the lumen continue for 4-8 hr and then droplets of protein are formed which remain in the cytoplasm. The length of time for active synthesis and secretion varies with the mode of stimulation. If the stimulation is caused by emptying of the gland this phase of the cycle lasts for about eight hours; with cholinergic stimulation the duration is only about four hours. The difference is presumably due to the fact that the lumen of the gland remains full of protein in the case of cholinergic stimulation. The importance of the cholinergic mechanism is unknown. It is possible that it acts as a fine control in regulating the production of protein and is the means whereby external stimuli can affect the amount of silk available for web-building.
After droplets of protein are formed in the cytoplasm, the rate of synthesis of protein decreases greatly. However, preliminary work on the composition of the amino acid pools suggests that the accumulation of the most prominent amino acids, alanine and glycine, continues until their levels are high. In glands in which active synthesis of new protein is under way, the level of these two amino acids is low, presumably due to high demand, whereas, in the resting state the levels of alanine and glycine are high. Thus, the gland is poised for action.
There are four experiments which independently suggest that the spider has information on the amount of silk available for web-building (Witt and Reed, 1965; Witt, et aL, 1968). Since the value of an incomplete web as a means of catching food is limited, the importance of this information is obvious. The experiments, details of which can be found in the references cited above, are (1) starvation, in which the spider makes a smaller, wider-meshed web; (2) additions of weights to the back of the spider, when a thicker but shorter thread is used in a full-sized web; (3) oral administration of physostigmine, when more protein is used to make a larger web, the increase being especially great in the sticky spiral; and (4) the burning out of radii (Koenig, 1951; Reed, this symposium). In the last case the spider replaces the radii for a while, but finally ignores the oversized angle and goes on to building the spiral. This experiment indicates that the spider may have a continuous feed-back of information on the supply of silk.
The debate about which gland supplies
76
David B. Peakall

FIG. 3. Cross-sections of the tail of the ampullate gland. Sections treated with DNase (0.1mg/ml) for 3 hr at room temperature. Sections were then stained with Azure B (40°, 3 hr, pH 4.0) and cleared with t-butyl alcohol, x 200
a. 10 min after stimulation.
b. 20 min after stimulation.
c. 60 min after stimulation.
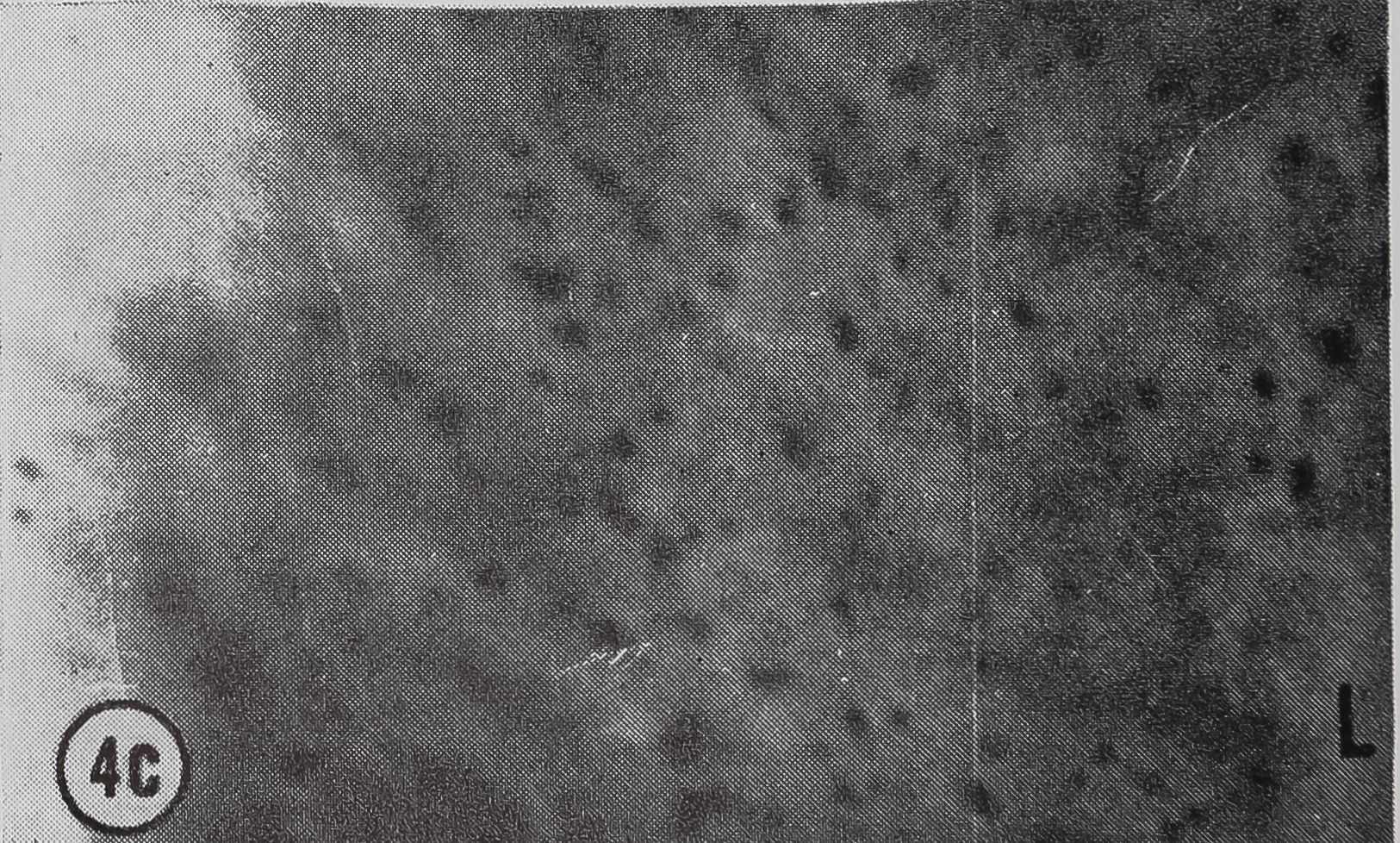
FIG. 4. Autoradiographs of a cross-section of a sin

gle epithelial wall of the tail of the ampullate gland. Five minute pulse of C14-orotic acid given at the time of stimulation. Sections coated with Ilford L4 emulsion, lightly stained with Azure B. L, lumen of gland, x 1200
a. 10 min after stimulation.
b. 20 min after stimulation.
c. 60 min after stimulation.




Synthesis of Silk
77

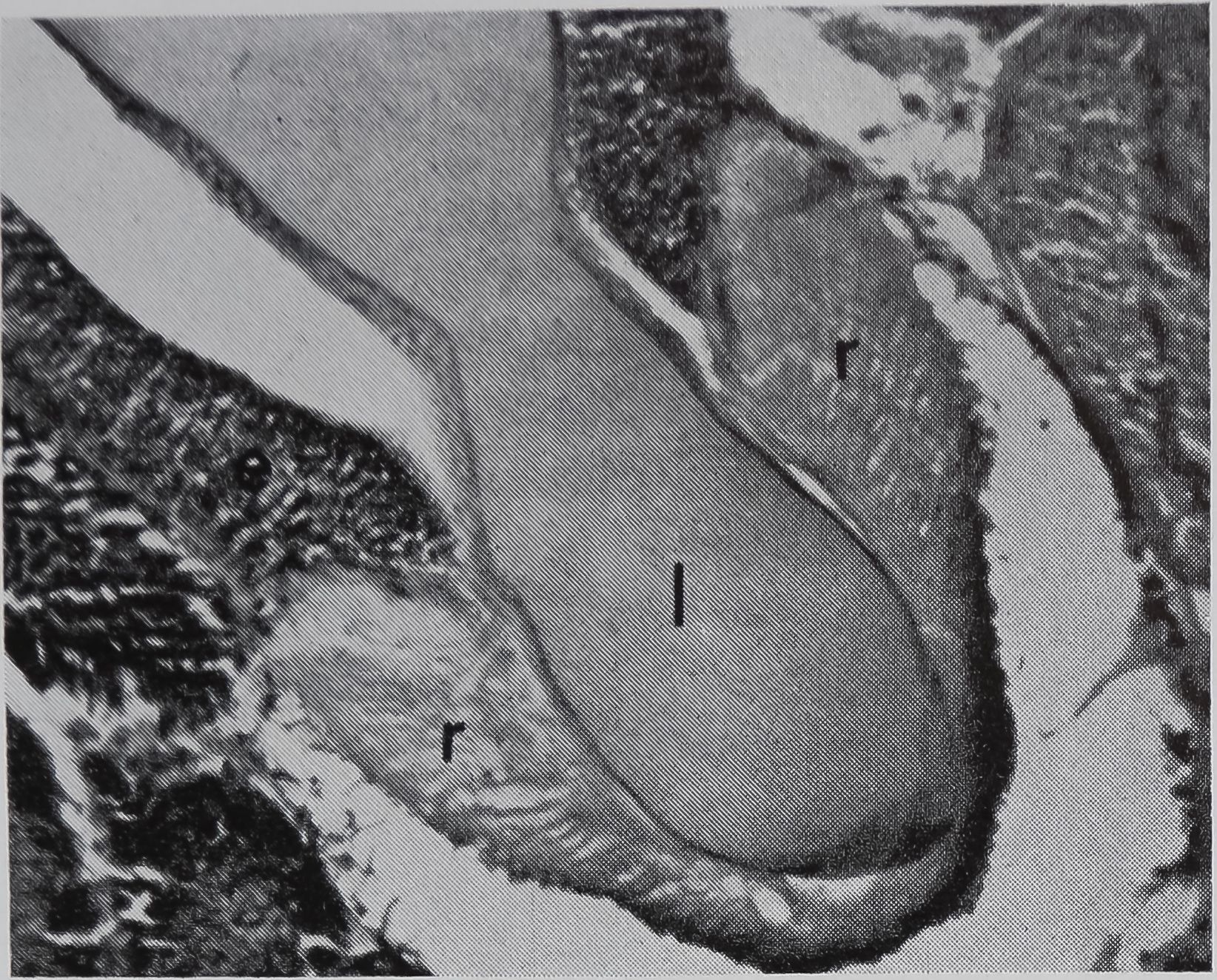
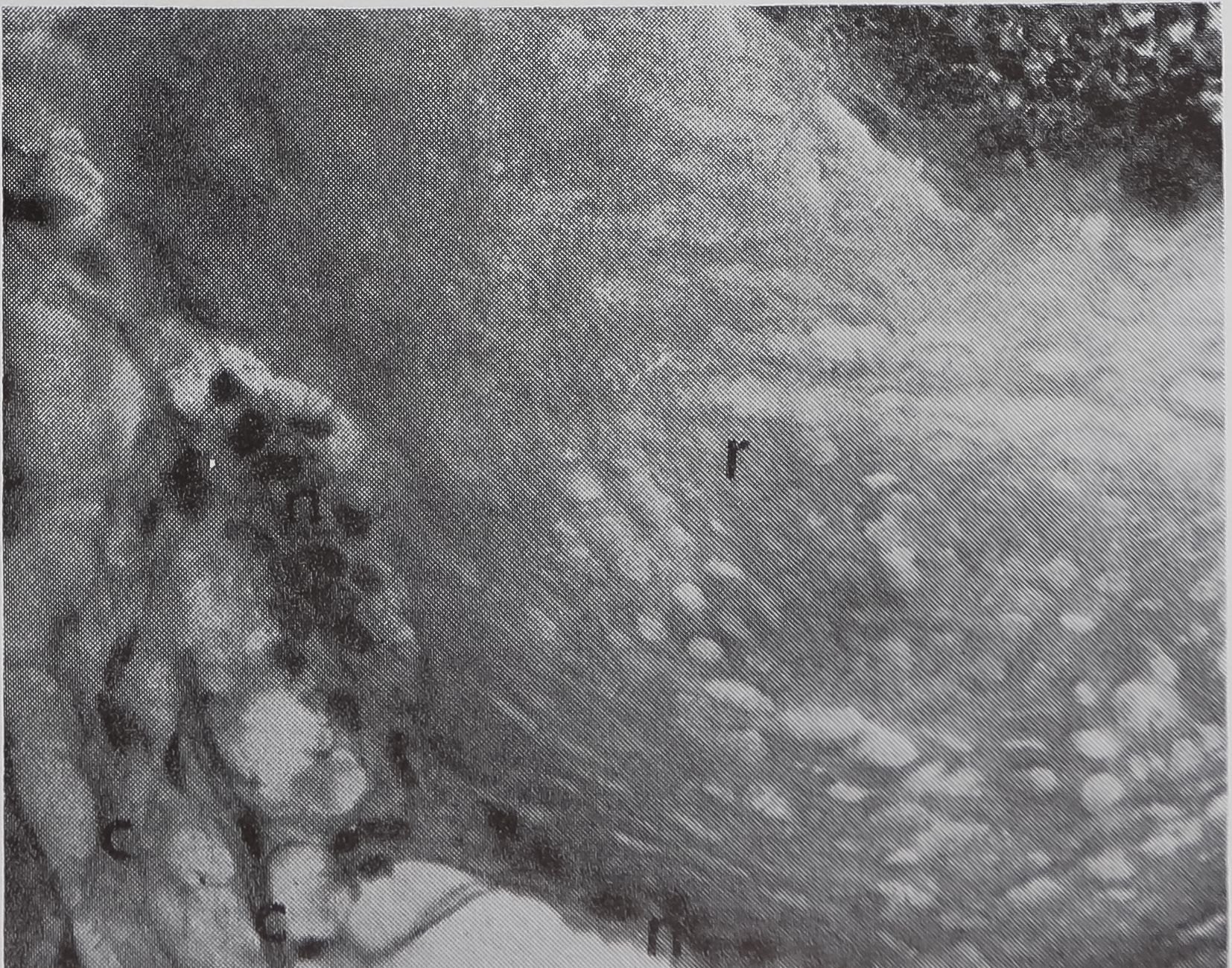
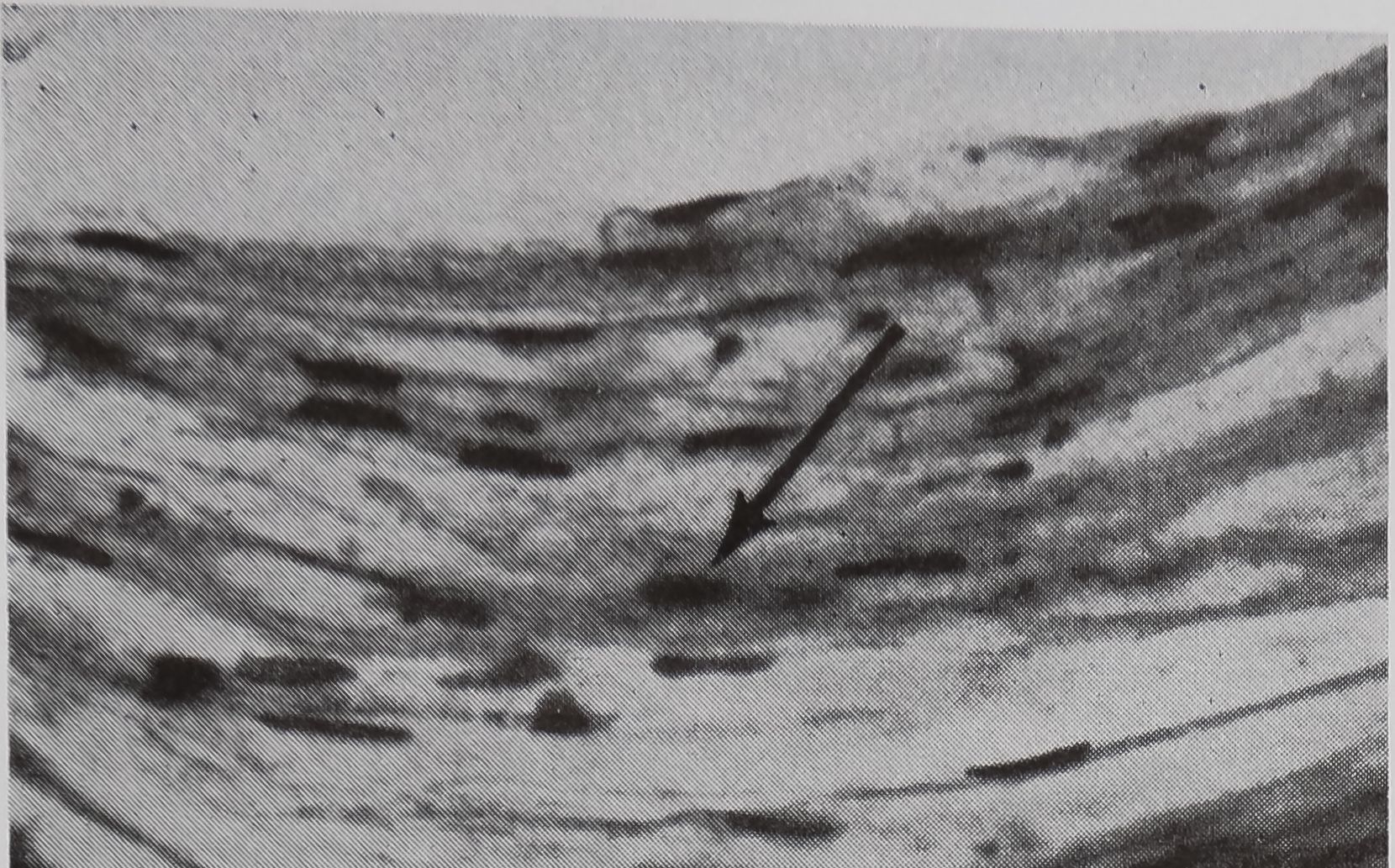
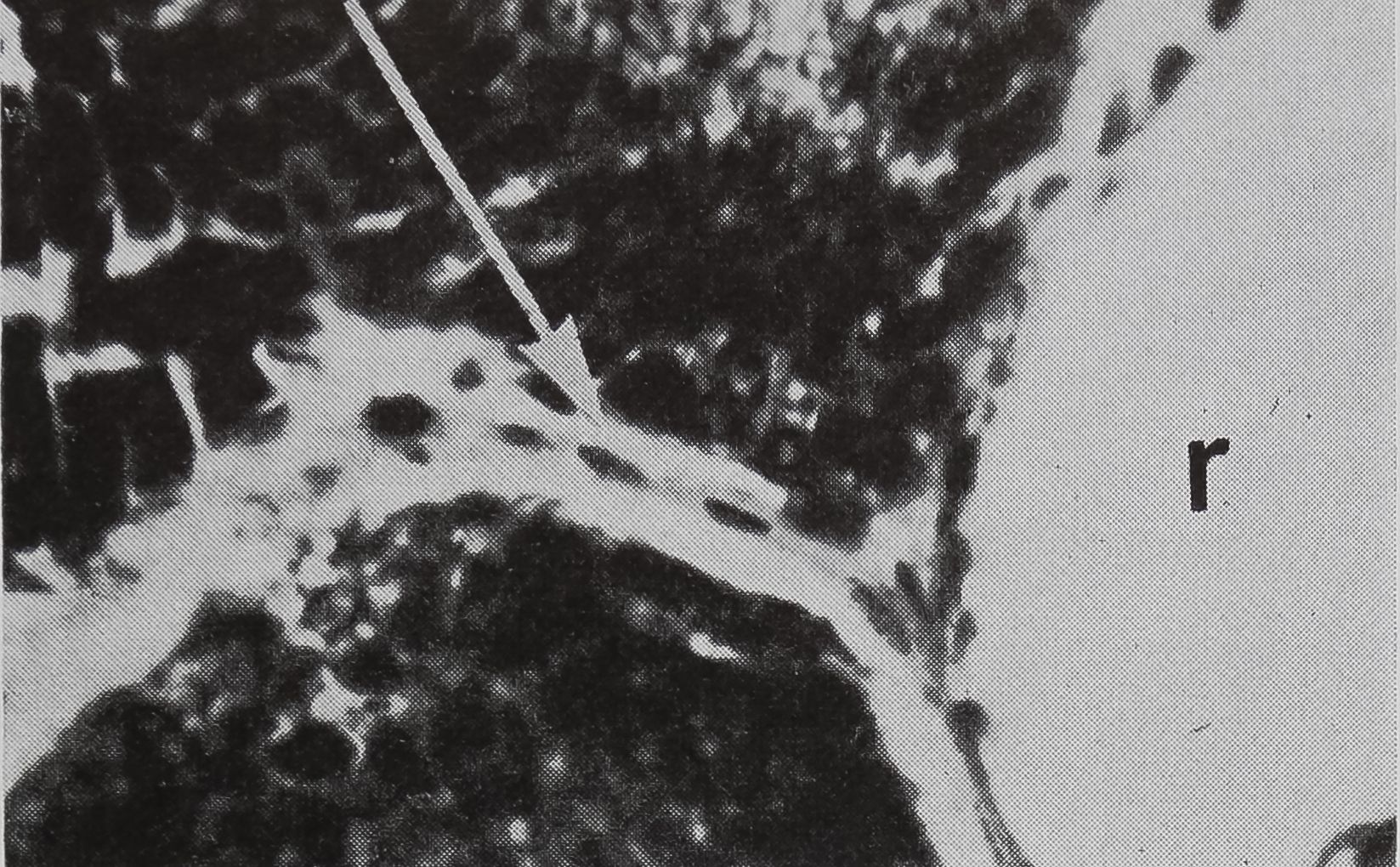
FIG. 5. Cross-sectional views of the ampullate gland showing a structure considered to be a receptor sensitive to the amount of silk stored in the gland. 1, lumen of gland; e, epithelium; r, receptor; t, tail of ampullate gland; n, nuclei at edge of receptor; c, connective tissue. Stained with hematoxylin and eosin.
a. Showing the receptor in contact with the lumen. X 200
the silk for the base-line of the sticky spiral does not affect the interpretation of these experiments to any considerable extent. If the material used for the base-line of the sticky spiral comes from the ampullate gland, then the importance of the feed-back of information is clear for all four experiments. Even in the last experiment, that of burning out radii, the value of the feed-back mechanism still remains, assuming that the provisional spiral comes from the ampullate gland. It is of interest that Koenig (1951) finds that the sticky spiral is minimal following the burning out of radii. This suggests that the same gland is involved both in constructing radii and forming sticky spirals, althoughBt is possible that the small spiral is due to disturbance of the spider by the experi-mentor.
The major question is the mechanism by which the feed-back of this information is mediated. None of the changes that occur in the cell during the cycle of secretion and synthesis that follows the stimulation of the gland appear to be related to the amount of silk present in the lumen. Moreover, whereas most of the silk is synthesized in the tail of the gland, it is stored
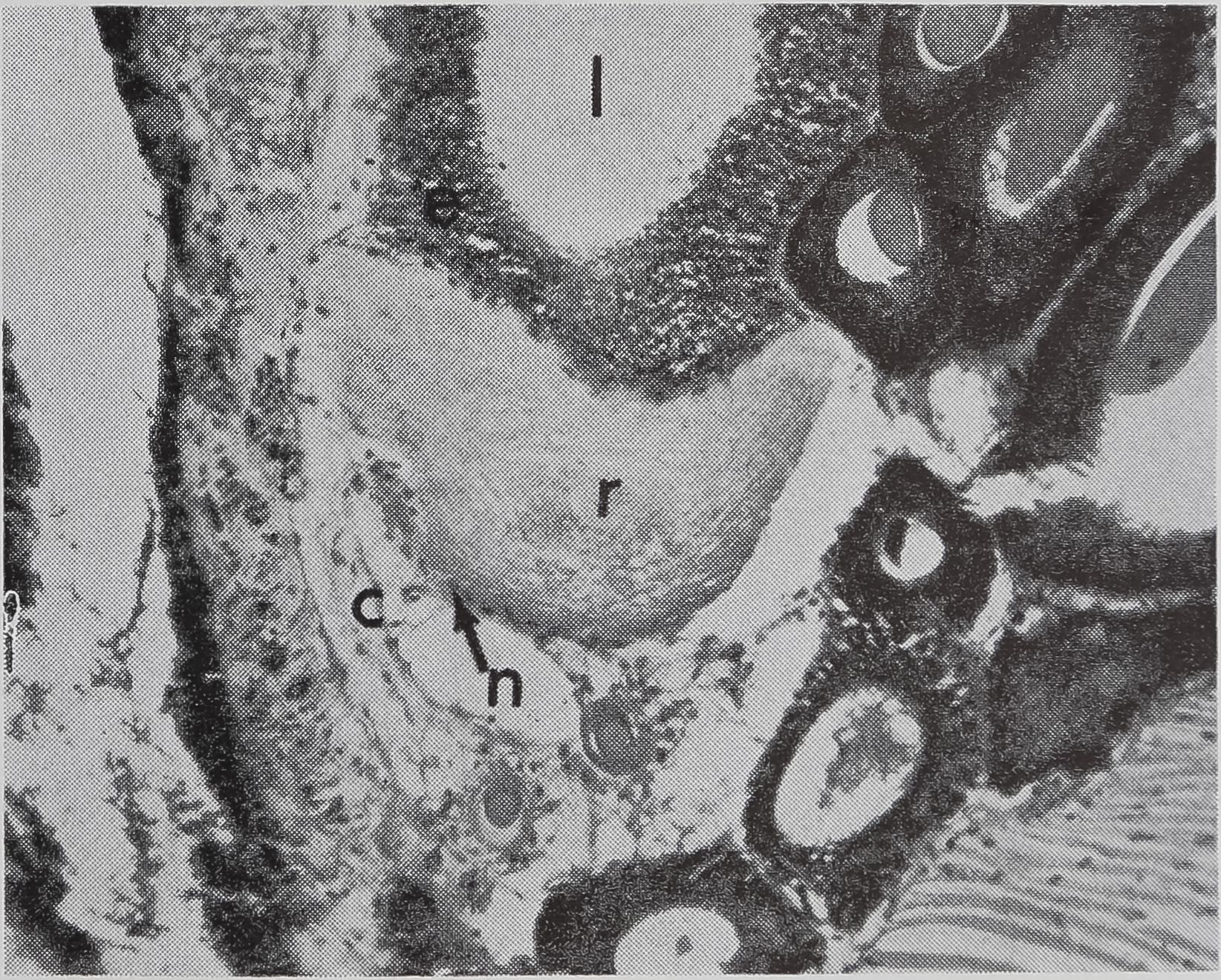
in the sac portion. Serial sections of the abdomen through the sac of the ampullate gland were examined. A structure which could be the receptor for the transmission of this information is shown in Figure 5a-c. In Figure 5a the “receptor” is in contact with the silk in the gland in a section of the lumen. The appearance of the “receptor’’ is very different from the normal epithelium in sections stained with hematoxylin, where the “receptor” is largely stained with eosin. The “receptor” has a striated structure and completely lacks the droplets of protein which are the predominant feature of the normal epithelial cells. Figure 5b is a photograph of another section of the same spider, 100 microns away from the section shown in Figure 5 a. At this stage, the receptor is not in contact

b. A section 100 ju, away from (a); receptor no longer in contact with lunien. x HO

c. Higher-powered view of part of (b). Peripheral nuclei and connective tissue can be seen, x 455
78
David B. Peak all

FIG. 6. Comparison of main nerve cord in the pedicel (6a) with the fiber attached to the receptor (6b). Both fibers show elongated nuclei. Stained with Trichrom. X300
with the lumen of the gland, but the peripheral nuclei and associated fibers can be seen. These features are more clear under higher magnification (Fig. 5c). Cross-sections of the coiled tail of the ampullate gland are also seen in Figure 5b. Staining with silver showed only poorly-stained fine fibers, which may represent the innervation of this structure. Staining with trichrome shows fibrous material with nuclei that were similar to main ganglia passing through the pedicel (Fig. 6a, b).
The structure described appears to have the necessary structural requirements to act as a biological transducer. The striated structure would be capable of transmitting pressure changes which could be changed to nerve impulses. In general, it is similar to a Pacinian corpuscle, a structure in mammalian skin and muscle which converts pressure into nerve impulses (Loew-enstein, 1960).
The orb web-building spider presents some unique opportunities for inter
disciplinary studies, some of which are illustrated in this symposium. The silk glands, as discrete organs producing a single structural protein, are a good model for the biochemist to study protein synthesis. The vital nature of these glands makes their role in the over-all economy of the animal of especial interest. The web produced by this structural protein makes a permanent, measurable record of a complex behavioral pattern. The pattern can be modified by many factors, a few of which affect the synthesis of silk directly. Such a complex operation as the construction of an orb-web must require the utilization of a great deal of the spider’s central nervous system. The relationship of changes in pattern in the orb-web to chemical changes in the spider’s brain might well be a fascinating and rewarding subject for study.
REFERENCES
Ambrose, E. J., C. H. Bamford, A. Elliott, and W. E. Hanby. 1951. Water-soluble silk: an a-protein. Nature 167:264-265.
Apstein, C. 1889. Bau und Funktion der Spinndriisen der Araneida. Arch. Naturg. 55:29-74.
Bell, A. L., and D. B. Peakall. 1969. Changes in fine structure during silk protein production in the ampullate gland of the spider (Araneus serica-tus). J. Cell Biol. (In press)
Blackwell, j. 1839. On the number and structure of the mammulae employed by spiders in process of spinning. Trans. Linnàean Soc. London 18:219-224.
Braunitzer, G., and D. Wolff. 1955. Vergleichende chemische Untersuchungen fiber die Fibroine von Bombyx mori und Nephila madagascariensis. Z. Naturforsch. 10b:404-408.
Comstock, J. H. 1948. The spider book. Comstock Publishing Co., Inc.
Koenig, M. 1951. Beitrage zur Kenntnis des Netz-baus orbiteler Spinnen. Z. Tierpsychol. 8:462-493.
Lenormant, H. 1956. Infrared spectra and structure of the proteins of the silk glands. Trans. Faraday Soc. 52:549-553.
Loewenstein, W. R. 1960. Biological transducers. Scientific American 203:98-108.
Peakall, D. B. 1964. Composition, function, and glandular origin of the silk fibroins of the spider, Araneus diadematus Cl. J. Exptl. Zool. 156:345-350.
Peakall, D. B. 1965. Differences in regulation in

Synthesis of Silk
79
the silk glands of the spider. Nature 207:102-103.
Peakall, D. B. 1966. Regulation of protein production in the silk glands of spiders. Comp. Bio-chem. Physiol. 19:253-258.
Peakall, D. B. 1968. Autoradiographic localization of acetylcholine in the ampullate gland of the spider. J. Pharmacol. Exptl. Therap. 160:81-90.
Peters, H. M. 1955. Ueber den Spinnapparat von Nephila madagascariensis. Z. Naturforsch. 10b: 395-404.
Sekiguchi, K. 1952. On a new spinning gland found in geometric spiders and its functions. Ann. Zool. Jap. 25:394-399.
Warburton, C. 1890. The spinning apparatus of
geometric spiders. Quart. J. Microscop. Sci.
Warwicker, J. O. 1960. Comparative studies of fibroins. 2. The crystal structures of various fibroins. J. Mol. Biol. 2:350-362.
Wilson, R. S. 1962. The control of the dragline spinning in the garden spider. Quart. J. Microscop. Sci. 103:557-571.
Witt, P. N., and C. F. Reed. 1965. Spider-web building. Measurement of web geometry identifies components in a complex invertebrate behavior pattern. Science 149:1190-1197.
Witt, P. N., C. F. Reed, and D. B. Peakall. 1968. A spider’s web. Problems in regulatory biology. Springer-Verlag, Heidelberg.