Images Collection
View this article in Search Friendly Plain Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
THERAPEUTIC AND TOXIC EFFECTS OF OUABAIN ON K+ FLUXES
IN RABBIT ATRIA
RICHARD S. TUTTLE, PETER N. WITT and ALFRED FARAH
Department of Pharmacology, State University of New York, Upstate Medical Center,
Syracuse, New York
Reprinted from The Journal of Pharmacology and Experimental Therapeutics Vol. 137, No. 1 July, 1962
Copyright © 1962 by the Williams & Wilkins Co.
Printed in U.S. A.
Reprinted from Tub Journal of Pharmacology and Experimental Therapeutics Voi. 137, No. 1 July, 1002
Copyright © 1062 by the Williams & Wilkins Co.
Printed in U.S. A.
8782
THERAPEUTIC AND TOXIC EFFECTS OF OUABAIN ON K+ FLUXES
IN RABBIT ATRIA1
RICHARD S. TUTTLE,2 PETER N. WITT and ALFRED FARAH
Department of Pharmacology, State University of New York, Upstate Medical Center,
Syracuse, New York
Received for publication March 12, 1962
The cardioactive glycosides have been variously reported to reduce (Wood and Moe, 1938), increase (Boyer and Poindexter, 1940) or not change (Hagen, 1939) myocardial potassium (K+) concentration. Earlier observations have shown that these glycosides reduced intracellular K+ in the heart and led to the hypothesis that the therapeutic effect of these drugs was causally related to a reduction in intracellular K+ concentration (HajdfH 1953; Hajdu and Leonard, 1959). This was based on evidencie from both isolated and intact myocardium. Changes in the rate of movement of K+ across the cell membrane have been reported under conditions where a net loss of intracellular K+ occurs. These studies have shown that the glycosides in sufficient concentration block active transport of K+ into the cells (Schatzmann, 1953; Schreiber, 1956; Rayner and Weatherall, 1957) and some-?’ times increase K+ efflux (Vick and Kahn, 1957).
However, recent studies on the effects of the cardioactive glycosides on intracellular K+ indicate that the reduction of K+ may be a “toxic” effect of these drugs which is unrelated to the “therapeutic” effect on contractility. Kühns (1959) and more recently Tuttle et al. (1961) have shown that intracellular K+ is either increased or unchanged after exposure to therapeutic concentrations of the glycosides. Similar results have been obtained with myocardium exposed to the glycosides in situ (Tuttle et al.,, 1961). In this case a “therapeutic” concentration was calculated as 50% of the dose producing irregularities in the electrocardiogram. Moreover, in the isolated myocardium where the loss of the frequency-force relationships is used as an indication of toxicity, intracellular K+ is not reduced
1 Supported by a grant from the American Heart Association, Inc.
2 Public Health Service Post-Doctoral Fellow. Present address: Masonic Foundation for Medical Research, Utica, N. Y.
at the ¡onset of these symptoms of toxicity (Tuttle et al., 1961). These observations are based on the terminal analysis of myocardium for K+ and sodium (Na+) and have added to the increasing evidence against the hypothesis that a low intracellular K+ concentration is causally related to the positive inotropic effect of the digitalis drugs.
Since a transient change in K+ flux cannot be detected by terminal analysis of K+ and since this may explain the discrepancies in the literature, it was of interest to determine the time course of changes in K+ fluxes occurring during exposure to therapeutic and toxic concentrations of the cardioactive glycoside, ouabain.
Methods. Isolated left atria from rabbits were a suitable tissue to study K+ fluxes and the inotropic effects of ouabain3 since the muscle walls are Less than 0.5 mm thick and do not contract spontaneously. The force of contraction developed by isolated atria is related to the stimulus rate and concentration of the cardiac glycoside, and such a relationship has been studied thoroughly in this laboratory (Tuttle and Farah, 1962). Both the “toxic” and “therapeutic” effects of ouabain are easily discernible from changes in the frequency-force relationships. Alterations in K+ flux during exposure to ouabain may be safely categorized as related to “toxic” or “therapeutic” effects.
To study the effect of stimulus rate and ouabain on K+ efflux, resting atria were placed in radioactive K+ Tyrode’s solution for 120 minutes, washed for 5 minutes in nonradioactive Tyrode’s solution and mounted on electrodes as described previously (Tuttle et al., 1961). The atria were then placed in muscle baths containing 5 ml of nonradioactive Tyrode’s solution, left quiescent for the first 70 minutes and then alternately stimulated for 10-minute periods at 0.1, 1.0 and 4.0 beats per second. At 2- or 5-minute intervals
8 We are grateful to S. B. Penick & Company for supplying the ouabain.
24
1962
OUABAIN EFFECTS ON K+ FLUXES
throughout tho experiment the bath solution was completely withdrawn into nalgene tubes and fresh nonradioactive solution added to the bath. The efflux of K42 from the muscles was reported for each period as the fraction of total K42 lost. This has been termed the “relative efflux” and is calculated as shown below:
K42 lost during a 2-min
. I. period beginning at Tx
relative efflux = –;—-¡— – .
available K42 in the atria at Tx
The use of relative efflux permits comparison of the efflux at different periods of the washout experiment since this figure is independent of the decreasing intracellular K42 concentration. Relative efflux became constant after a 40-minute rest period and evaluation began only after that. In a second series of experiments ouabain was added to the bath solution in various concentrations after control values had been obtained for the efflux at rest and during stimulation. The ouabain was added to the bath solution at the beginning of the 30-minute test period dividing the control from the drug studies. Where the effects of a“toxic” concentration of ouabain were studied, this period was reduced to 15 minutes to prevent too severe depression of the atria. The sampling period was of 5 minutes duration in these experiments. It may be stressed that each atrium served as its own control for the effects of ouabain. Tension was recorded throughout the experiment (isometric strain gauge measurements) so that the effects of ouabain on contractility could be recorded.
The uptake of K42 was studied by two methods. In the first, the atria were placed in a muscle bath at 37.5°C directly over a solid scintillation crystal. Radioactive Tyrode’s solution flowed past the atria at a rate of 5 ml/minute and the uptake was recorded continuously on a linear recorder. When desired, ouabain in various concentrations was added to the solution while the uptake was recorded. In the second group of experiments the atria were placed in muscle baths (either 5- or 100-ml baths) kept at 37.5°C and containing radioactive Tyrode’s solution and at the end of either 10 or 20 minutes the muscles were removed and radioactivity recorded. In these latter experiments the effect of stimulation on K42 uptake was also investigated. Total K+ and Na+ were determined at the end of the uptake periods.
A well type scintillation counter (Baird Atomic, Model 810) was used for determining the radioactivity in the solid and liquid samples. The usual corrections for background, decay and geometry were applied to all counts.
Results. Figure 1 relates the effects of dififer-
25
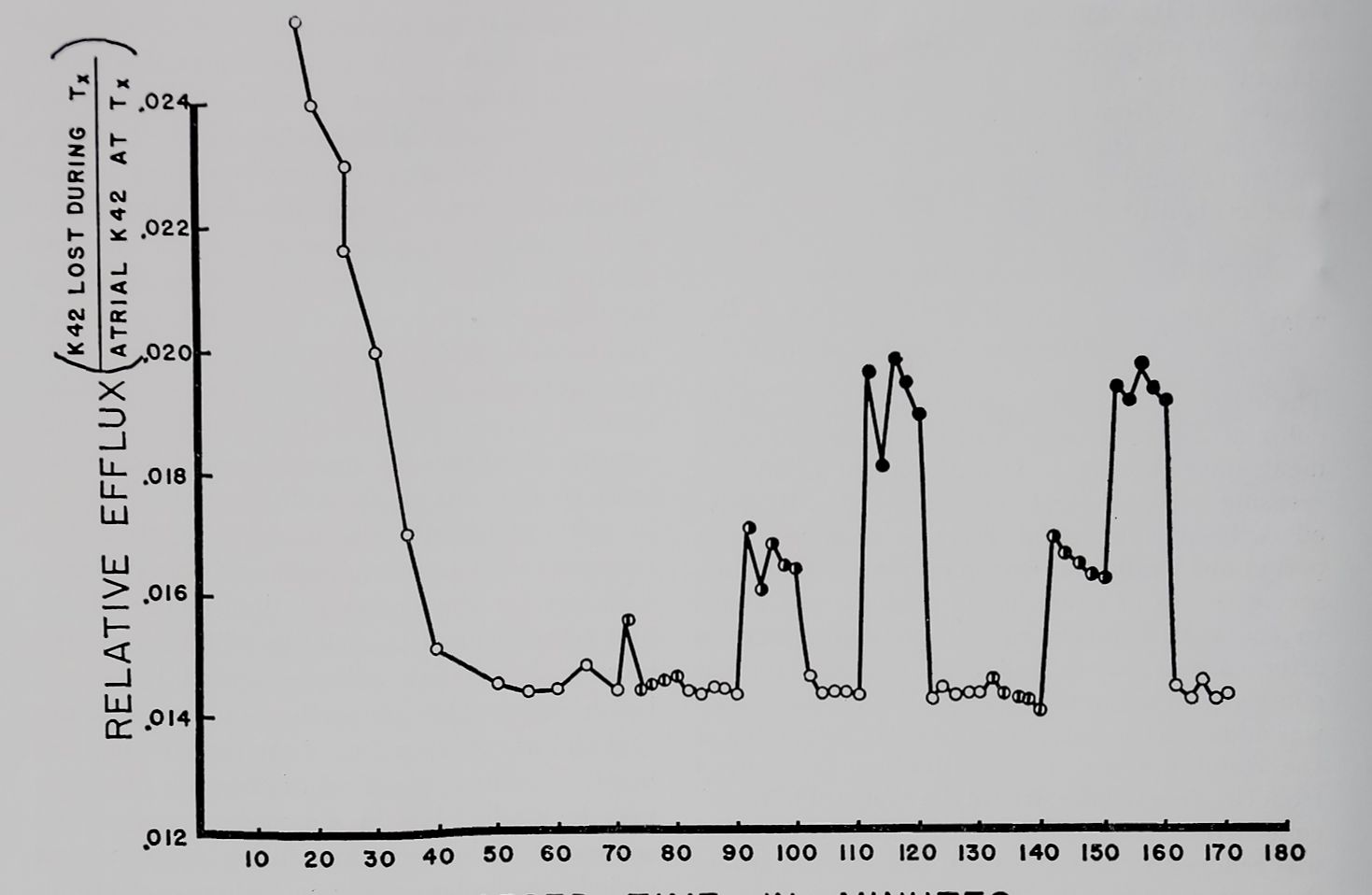
ent stimulation rates to the relative efflux of K42 from isolated rabbit atria. There was a considerable decrease in the relative efflux in the first 50 minutes of the experiment; however, this leveled out and remained constant for the duration of the experiment unless stimulation was begun. A biphasic curve for the efflux of K42 from myocardium has been reported by other investigators (Schreiber, 1956; Rayner and Weatherall, 1957). Differences in the time course are a function of the technique used for washing out the isotope. A constant flow technique, preventing simultaneous uptake, would accelerate both components of the efflux curve. However, in our experiments the washout solution was changed only every 2 minutes and this probably accounts for the prolonged time course of the first component of the relative efflux curve. The increase in relative efflux produced by stimulation was roughly proportional to the stimulus rate and was independent of the time of measurement. Therefore, the effect of drugs on the efflux may be studied late in the washout period and compared with the efflux during early control periods. In similar experiments where each stimulation period was of 30 minutes duration, the relative efflux at each stimulation rate remained constant and above the level of the efflux during rest. As soon as the atrium was at rest again (after a period of stimulation) relative efflux returned to rest level.
Table 1 is a statistical analysis of the loss of K42 at 1.0 and 4.0 beats per second and after exposure to either 10-6 or 5 X 10-8 M ouabain. The results contained in table 1 were obtained from similar experiments as in figure 1. Although the relative efflux values during rest and stimulation were not exactly comparable it should be remembered that each drug washout experiment was compared against its own control period prior to the addition of the drug. In figure 1, K42 loss was increased with increasing stimulus rate. However, in these experiments (table 1) this was not as pronounced as in those in figure 1 due to our use of 5-minute sampling periods instead of 2-minute periods. The initial loss of K42 which was sometimes higher has not been included in these calculations. 5 X 10“8 M ouabain did not change the loss significantly in resting atria (D), but did reduce the rate (below the 1 % probability level) in atria contracting at 1.0 and 4.0 beats per second (E and F). The
26
TUTTLE ET AL.
Vol 137
ELAPSED TIME IN MINUTES
Fig. I The relative loss of K42 from isolated left atria of rabbits.
Atria exposed for 2 hours prior to zero time to radioactive Tyrode’s solution and K42 washed out in nonradioactive Tyrode’s solution. Open circles: loss at rest; split circles: at 0.1/sec; half and solid circles at 1.0 and 4.0/sec, respectively.
Abscissa: time of incubation in nonradioactive Tyrode’s solution with sampling every 2 minutes. Ordinate: the relative efflux during each 2-minute period. Data from one atrium representative of a number of similar experiments.
TABLE 1
Changes in the rate of loss of Ki2 from isolated rabbit atria at rest and at different stimulus rates under the influence of a lo’i^mherapeuti& and a high {toxic) concentration of ouabain
Observe the highly significant decrease in efflux from beating atria at the low ouabain concentration in contrast to the highly significant increase at the high ouabain concentration.
| Group | Ouabain M | Rate (stim-uli/sec)H | Nö’.-M”’”
Observations |
!No. of Atria | Mean Relative K42 Efflux/5 min without First 5 min, and S.E.M. | Significantly Different from (P |
| A | — | 0 | 30 | 6 | 0.049 =fc 0.0018 | |
| B | — | 1 | 30 | 6 | 0.055 ± 0.0018 | E |
| C | — | 4 | 30 | 6 | 0.057 =fc 0.0039 | F |
| D | 5 X 10~8 | 0 | 24 | 6 | 0.041 ± 0.0019 | |
| E | 5 X 10“8 | 1 | 24 | 6 | 0.039 d= 0.0023 | B |
| F | 5 X 10“8 | 4 | 24 | 6 | 0.037 ± 0.0014 | C |
| G | — | 0 | 25 | 5 | 0.062 ii 0.0045 | |
| H | — | 1 | 25 | 5 | 0.063 ± 0.0022 | K |
| I | — | 4 | 25 | 5 | 0.064 d= 0.0029 | L |
| J | 10″fl | 0 | 10 | 5 | 0.047 =fc 0.0200 | L |
| K | 10“6 | 1 | 10 | 5 | 0.085 =fc 0.0116 | H |
| L | 10~6 | 4 | 10 | 5 | 0.093 =fc 0.0142 | I,J |

1962
OUABAIN EFFECTS ON IC+ FLUXES
27
tension ohanges reoorded from these atria indicated a “therapeutic” positive inotropic effect at 4.0 beats per second and no change in contractility at 1.0 beat per second (Tuttle and Farah, 1962). The terms “toxic” and “therapeutic” concentration used in this text are derived from studies already reported by the authors (Tuttle et al., 1961; Tuttle and Farah, 1962). That concentration of ouabain which reduces intracellular K+ and contractile tension at physiological heart rates and increases tension at lower than physiological heart rates is considered as being “toxic.” That concentration of ouabain which maintains intracellular potassium and increases tension at physiological heart rates is considered as being “therapeutic.” These criteria are applied in this present study.
The lower part of table 1 shows the effects of a higher “toxic” concentration of ouabain on the loss of K42. The atria were stimulated during the exposure period to 10“6 M ouabain only half as long as in the previous experiments (30 minutes) because of the rapid decrease in excitability and deterioration of contractility. 10~6 M ouabain significantly increased the loss
of K42 from the atria during activity. Associated with the increased loss at each stimulus rate was a decreased contractility at 4.0 beats per second and an increased contractility at 1.0 beat per second. This is considered to be a ‘‘toxic” effect (Tuttle and Farah, 1962).
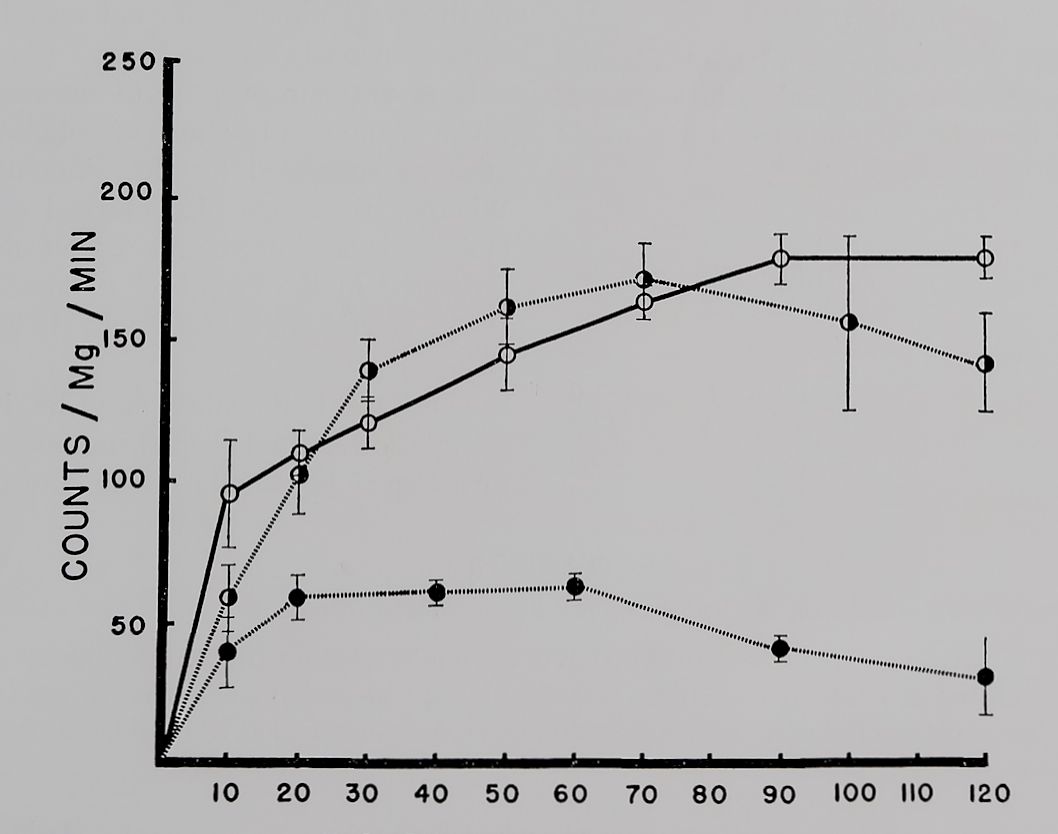
The uptake of K42 by quiescent atria is plotted in figure 2. During the first 10 to 20 minutes the atria gained K42 at more than twice the rate of uptake during the later periods. From the loss measurements and from the size of the interstitial space we consider that the first 5 to 10 minutes of the rapid exchange represents both interstitial and intracellular accumulation of the isotope. Complete exchange of intracellular K+ with extracellular K42 occurred at 120 minutes.
In the uptake studies of quiescent atria a somewhat higher concentration (10~7 M) of ouabain was used as the “therapeutic” concentration. This was the result of previous observations which have shown that resting atria are less sensitive to ouabain than contracting ones (Tuttle et al., 1961). Figure 2 shows that this concentration initially increased slightly and then did not change the rate of uptake of K42 by

INCUBATION TIME IN MIN
Fig. 2. The uptake of K42 by atria at rest.
The muscle baths were mounted directly over a solid scintillation crystal with radioactive K+ flowing past at a rate of 5 ml/min. Uptake recorded continuously by a linear recorder.
Abscissa: time of incubation in K42 Tyrode’s solution. Ordinate: uptake in counts/mg of tissue/min. Open circles: control; solid and half circles during exposure to 10~B and 10~7 M ouabain, respectively. Six atria in each group. Vertical lines are the standard errors of the mean. Observe the significantly lower uptake of K42 by atria exposed to 10“5 M ouabain when compared to controls or 10-7 M ouabain group.
28
TUTTLE ET AL.
Vol 197
quiescent atria. Some decrease in intracellular K42 was observed in atria at the end of 120 minutes exposure to ouabain. For similar reasons as described above, 10~6 M ouabain was chosen to produce a “toxic” effect on quiescent atria. This concentration markedly reduced the uptake of K42 (fig. 2). The slow component of K42 uptake was most affected with little change in the fast fraction of uptake.
In atria contracting at 4.0 beats per second uptake was measured after 10 minutes exposure to radioactive K+ (table 2). The effect of two different concentrations of ouabain on K42 uptake was studied by preincubating the atria in ouabain for 10 minutes before exposure to the isotope solution which also contained ouabain. The lower concentration of ouabain may be considered as “therapeutic” as contractility was increased in 20 minutes by 32%. Under these conditions a significantly greater amount of K42 had been taken up after 10 minutes when com-
TABLE 2
Effects of ouabain 5 X 10“8 and 10~6 M on the uptake of Ki2 into atria beating at 4/second at 87.5°C
Each atrium was incubated without stimulation in a nonradioactive Tyrode’s solution for 20 minutes and then for 10 minutes in 100 ml of Tyrode’s solution containing K42.
| Group | Ouabain M | No. of Observations | %K+ Exchanged in 10 min, Mean and S.E.M. | Signifi
cantly Different •””from (Pg.OlJ |
1. % Change in Con-föjtäpcpH* : “tility |
| A | 8 | 14.7 ± 1.24 | B | 0 | |
| B | 5 X 10~8 | 10 | 23.1 d= 2.If | A | I +32 |
| C | io-6 | 8 | 17.1 =b 1.34 | -69 |
pared to control rate. 10~° M ouabain decreased contractility by 69% (table 2) but had no detectable effect on K42 uptake. Figure 2 suggests that we would probably have measured a decreased uptake with a “toxic” concentration after longer periods of exposure to the drug and isotope.
Incubation at 28.0°C if compared to 37.5°C produced a frequency dependent positive inotropic effect. Table 3 shows the K42 uptake and total intracellular K+ at different temperatures. The lower temperature did not change intracellular K+ concentration significantly in the time measured although K42 uptake was significantly decreased. The addition of 10“6 M ouabain to atria at 28.0°C increased contractility still further but had no additional effect on reducing the uptake of K42.
Discussion. These new data together with those reported previously (Tuttle et al., 1961) permit us to summarize the effects of different concentrations of ouabain on contractility, intracellular K+concentration and K+ fluxes in the rabbit atria. It should be kept in mind that by nature of the isotope techniques the conditions of these experiments are not exactly similar to those previously reported.
It is not our purpose to discuss the significance of the two fractions of potassium in rabbit atria as suggested by the different flux rates. Whether these are physiological or anatomical moieties is not known and explicit discussions are presented in the literature (Solomon and Gold, 1955; Harris and Steinbach, 1956; Schreiber, 1956).
The fact that efflux may be increased by stimulation is not surprising as an increased turnover of K+ should accompany the increased
TABLE 3
The effects of temperature on the uptake of K42 by stimulated rabbit atria with and without drug
Each group of atria was incubated in 5 ml K42 Tyrode’s solution for 20 minutes at either 37.5 or 28.0°C and stimulated at 1 beat per second. Ouabain 10“6 M was added to another group treated in the same manner. Total intracellular K+ and radioactivity were determined at the end of 20 minutes. Corrections were made for 26% interstitial space.
| Group | Temperature
°C |
Ouabain M | No. of Observations | mEq K/kg Fiber Weight, Mean and S.E.M. | % K Exchanged in 20 min, Mean and S.E.M. | % Change in Contractility |
| A | 37.5 | 0 | 4 | 48.9 ± 3.8 | 30.6 ± 3.4 | 0 |
| B | 28.0 | 0 | 4 | 53.9 =b 2.5 | 17.6 d= 1.7 | +300 |
| C | 28.0 | 10“6 | 4 | 47.6 ± 2.6 | 16.7 =fc 3.1 | +350 |
% Exchange in groups A and B were significantly different with P = .016. % Exchange in groups A and C were significantly different with P <= .022.
1962
OUABAIN EFFECTS ON K+ FLUXES
29
number of action potentials. There are two explanations as to why the efflux did not increase in direct proportion to the increased rate of stimulation. A 1:1 relationship of stimulus rate to efflux would be expected if each action potential caused the same amount of K+ efflux. However, in studies of K+ flux in single muscle fibers Hodgkin and Horowicz (1959) reported that the flux per beat was reduced as stimulus rate increased. A similar situation may occur in myocardium. It is also possible that the actual efflux at 4.0 beats per second was 4 times the efflux at 1.0 beat per second but due to diffusion delay in the interstitial space, a fraction of K+ lost at each beat was returned to the intracellular space. We therefore would only be able to measure the difference between what actually left the intracellular space and what was returned during the same period of stimulation. The fact that we consistently were able to measure a significant relationship between the rate of stimulation and K+ efflux in the 2-minute sample period experiments (fig. 1) and not in the 5-minute sample periods (table 1) suggests that some return of isotope to the intracellular space occurred concomitantly with the efflux. Therefore, our measurements of the rate of loss of K42 from the muscle and uptake into the muscle actually represent a composite of efflux and influx. The more frequently the outside solution is changed, the more representative the measurements become of true efflux.
The situation is more complex in the uptake studies. As the atria gain K42 a greater percentage of intracellular K+ becomes radioactive so that any change in efflux rate would have significant effects on intracellular K42. A large change in efflux rate would have great effects on intracellular K42 which could be misconstrued as a change in influx rate.
From other studies (Tuttle et al., 1961) it is known that rabbit atria lose K+ and gain Na+ in Tyrode’s solution and are therefore not in equilibrium. Though the contractility does not change during this period of loss of K+ and gain of Na+, the factors which maintain K+ equilibrium must be impaired. The net loss of K+ has sometimes been reported to be due to increased efflux rather than a decreased influx. Rayner and Weatherall (1957) calculate a 2% net loss of K+ per hour due to efflux.
Previous studies have shown that when 5 X 10“8 M ouabain was added, the slow loss of
K+ was prevented; simultaneously contractility was increased. Under similar conditions of drug concentration and contractility changes, these atria show a decreased loss as well as an increased uptake of K42 when compared to untreated atria. It is difficult to determine whether our figures indicate a change in one or both fluxes due to reasons already discussed. We propose that a therapeutic concentration of ouabain maintains intracellular K+ concentration in rabbit atria by probably reducing K+ efflux and/or increasing influx.
A “toxic” concentration of ouabain had, together with its negative inotropic effect, a depleting action on intracellular K+ concentration (Tuttle et al., 1961). One explanation for this effect rests on the demonstration that K42 uptake, if observed for sufficient time, is reduced by a high concentration of ouabain. This action may not be observed during early periods of toxicity as indicated by a decrease in excitability and contractility. The observation that isometric tension decreased at a time when no effect on K+ uptake was observed supports the earlier conclusion that K+changes are unrelated in time to changes in contractility (Tuttle et al., 1961). Klaus et al. (1961) have published their results in abstract form and come to similar conclusions. The increased loss of K42 after exposure to a toxic concentration of ouabain could be explained entirely by the block in K42 uptake during the sampling periods. A constant flow analysis of K42 loss froH atria exposed to “toxic” and “therapeutic” concentrations of ouabain may be indicated. This would reduce problems of separating influx from efflux as encountered in this study.
The experiments at low temperatures show that the effects of cold and toxic ouabain concentrations on K+ exchange are in the same direction but not cumulative. As both cold and ouabain have a positive inotropic effect and these are additive, it is again apparent that neither intracellular K+ concentration nor K+ fluxes are causally related to the positive inotropic effects.
SUMMARY
Previous experiments with stimulated rabbit atria have indicated that “therapeutic” positive inotropic concentrations of ouabain maintain intracellular K+ in vitro and produce no change in vivo. “Toxic” negative inotropic concentrations
30
TUTTLE ET AL.
Vol 137
on the other hand decrease intracellular K+ concentration. Neither the toxic nor therapeutic effects on K+ as determined by terminal analysis of the tissue could be correlated with changes in excitability or contractility. This experiment was undertaken to measure changes in K+ fluxes during exposure to “toxic” or “therapeutic” concentrations of ouabain.
In isolated rabbit atria “therapeutic” concentrations of ouabain decreased K42 loss as well as increased uptake of radioactive potassium. This may explain both the maintenance and increase in intracellular K+ observed by other investigators. “Toxic” concentrations of ouabain blocked K42 uptake and this alone may explain the effects of digitalis on intracellular K+concentration. However, the inhibition of K+ uptake occurred later than the negative inotropic effect on contractility. This supports the previous assumption (Tuttle et al., 1961) that changes in intracellular K+ concentration or fluxes brought about by ouabain are not causally related to the inotropic effects of the drug.
REFERENCES
Boyer, P. K. and Poindexter, C. A.: Amer.
Heart J. 20: 586, 1940.
Hagen, P. S.: This Journal 67: 50, 1939.
Hajdu, S.: Amer. J. Physiol. 174: 371, 1953. Hajdu, S. and Leonard, E.: Pharm. Rev. 11: 173, 1959.
Harris, E. J. and Steinbach, H. B.: J. Physiol.
133: 385, 1956.
Hodgkin, A. L. and Horowicz, P.: J. Physiol.
145: 405, 1959.
Klaus, W., Kuschinsky, G. and Luellmann, H.: Biochem. Pharmacol. 8: 37, 1961.
Kühns, K.: in Herzinsuffizienz und Digitalis Wirkungen, p. 108, Springer-Verlag, Berlin, 1959.
Rayner, B. and Weatherall, M.: Brit. J. Pharmacol. 12: 371, 1957.
Schatzmann, H. J.: Helv. physiol, acta 11: 346, 1953.
Schreiber, S. S.: Amer. J. Physiol. 185: 337, 1956. Solomon, A. K. and Gold, G. L.: J. gen. Physiol.
38: 371, 1955.
Tuttle, R. S. and Farah, A.: This Journal 135: 142, 1962.
Tuttle, R. S., Witt, P. N. and Farah, A.: This Journal 133: 281, 1961.
Vick, R. L. and Kahn, J. B., Jr.: This Journal 121: 389, 1957.
Wood, E. H. and Moe, G. K.: Amer. J. Physiol. 123: 219, 1938.