Images Collection
View this article in Search Friendly Plain Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
Reprinted from Ecology
Vol. 55, No. 2, Early Spring 1974
pp. 317-328
Made in the United States of America
VERTICAL STRATIFICATION IN ORB-WEB SPIDERS
( ARANEID AE, ARANEAE) AND A CONSIDERATION OF OTHER
METHODS OF COEXISTENCE1
Frank Enders2
Division of Research, North Carolina Department of Mental Health and
Department of Zoology, North Carolina State University, Raleigh 27611
Abstract. Measurements of the web height and counts of the number of webs on randomly
selected plots in lespedeza fields reveal that immature Argiope aurantia and Argiope trifasciata,
very large araneid spiders, place their webs at different heights. In late summer this vertical
stratification disappears, while |||e numbers of the two species on plots become positively
correlated. Coexistence of the two spider species may depend in part upon the usual occurrence
of high mortality during the immature stages. Invasion of webs by araneids is reported as
possible competitive interference. The two largest species of European araneids also show
vertical stratification only as immatures. However, the niches of most Polish Araneus specief
in fields seem differentiated by parameters related to prey size: season of breeding and size
differences between species.
Key words: Behavior; interference; niched
Introduction
Cody (1968) showed that species of grassland
birds coexist by a combination ofepecializations both
vertical and horizontal in use of food and use of
space. Horizontal space may be a dummy variable
for habitat or other subtle differences in the needs
of the species. Temporal separation completes Cody’s
exhaustive list of schematic parameters of the niche.
His success in characterizing bird niches using such
schematic parameters ypggests that similar analyses
of “communities” of species of other taxa may pro-
vide a framework for understanding the natural
history of such groups.
Slobodkin (1961) suggested that predation must
permit the coexistence of more prey species than that
allowed by competition foç-a limiting resource. Paine
(1966, 1971) demonstrated that horizontal space
limits sessile iptertidal organisms, so that predation
upon the dominant competitor increases the number
of species that can coexist in a particular area. Rick-
lefs and O’Rourke (1973) consider the possibility
that appearance to predators may be treated as
another parameter of niche space, for sessile, cryptic
animals.
The use by web-building spiders of fixed webs
for foraging can provide clearcut data for investiga-
tions of the use of space. In most of North America,
two species of Argiope, a genus of large orb-web
spiders, coexist in fields: Argiope aurantia Lucas
(the “garden spider”) and Argiope trifasciata (For-
skal) (Levi 1968). These species overlap greatly in
the range of habitats (Fitch 1963, Enders 1973) and
in phenology (Muma and Muma 1949, Fitch 1963,
1 Received January 30, 1973; accepted August 13, 1973.
2 Current address: Department of Zoology, University
of Texas, Austin 78712.
spider; survivorship; web.
Enders 1973). These two species are also similar
in body size (Kaston 1948, Levi 1968), the size and
general appearance of the web and fangs (the trophic
apparatus), and the prey actually taken (Bilsing
1920). Thus, the two species actually coexist in most
field-type habitats, while apparently using the same
prey resource. Vertical stratification or a predation
effect would be necessary to allow their coexistence.
During a study of web site selection, I found both
species abundant in stands of sericea lespedeza (Les-
pedeza cuneata), a dicot perennial whose stems die
back each year. As the spiders were abundant there,
I was able to investigate the height at which webs
of the two Argiope species are placed and the absolute
numbers of each species. On occasion, the species
reach a similar abundance in more natural vegetation
(Enders 1®73). I noted invasion of other spiders’
webs, while observing marked individuals of various
species; the behavior can serve as the proximate
mechanism of the spatial separation of niches. The
annual decline in numbers of the two Argiope species
I observed suggests that, within a habitat, these two
competitors can coexist as adults because of high
mortality in the vertically stratified immature stages.
To determine whether coexistence via a predation
effect (Slobodkin 1961) was common among araneid
spiders, I then analyzed the season of breeding, the
adult size, and the stratum of vegetation used by a
group of ten species of the genus Araneus found by
Luczak (1963) in stands of heather with young pines.
Method
Random sampling of numbers and
location of Argiope webs
I chose the five largest adjacent road cuts along
U.S. Highway 1 Bypass northwest of Raleigh, North
Carolina. Despite the apparent uniform growth of
FRANK ENDERS
Ecology, Vol. 55, No. 2
Table 1. Correlations between no. of Argiope aurantia and A. trifasciata. Correlation coefficients calculated between
the no. of webs per m2 at the N randomly selected locations. No. of plots searched 2N in Aug. and 3N in Sept.
Numbers of the two species become positively correlated by late summer
Dates of sampling
May June July August September
1970 30/6-2/7/70 26/7-13/8/70 24/8-29/8/70 29/9-6/10/70
1971 26/5-3/6^1 3W6-2/g71 2/8-4/8/71 31/8-5/9/71
Correlation coefficients c -.04 .13 .12 .48** .27**
Both 1970 and 1971 data , N 29 93 158 143 79
c =: correlation coefficient.
N = number of locations.
** Correlation statistically significant at the .01 level.
sericea lespedeza (80% cover, planted about 10 years
ago), these areas had additional plants, mainly intru-
sive herbaceous “weeds” in the first four meters from
the road’s edge, including, in descending abundance,
Lactuca, Oenothera, Aster, Ambrosia, Rubus, and
Phytolacca. The shoulder of the highway and a
distance of two meters up the road cuts were covered
by Kentucky Tall Fescue Grass (Festuca sp.^ and
were mowed every month. Occasional trees, prin-
cipally Loblolly Pine (Pinus taeda), were present,
especially near the upper edge of the road cuts.
The total length of the areas sampled was 845 m,
excluding parts where the ditch at the edge of the
road wap concrete; the width averaged 25 m. At
monthly intervals, the length of the areas was system-
atically sampled, with a random start, by transects
taken up the slope of the road cuts. Dates of sampling
are given in Table 1. Monthly sampling began 1
week after the young Argiope aurantia were last to
be found in cocoons in order to find the maximum
number of this species on webs and ended in Septem-
ber to avoid the heavy mortality from frost in
October. In 1971 samples were taken from only the
two largest road cuts, 660 m long.
At each transect I searched successive plots of
1 m2 for spider webs. Taking the ditch as zero, the
first plot was between the roadside ditch and 2 m
towards the road, on alternate transects between 0
and -1 m, or between -1 m and -2 m. Since the
next meter of vegetation up the slope was trampled
during the search for webs, the lower edge of the
next plot was located 1 m up slope from the previous
plot (on alternate transects 1-2 m or 0-1 m). The
last plot searched was entirely within the lespedeza
which had a clear separation from adjacent forest.
To find webs, I first looked along the top of a plot
and also underneath, without disturbing the vegeta-
tion. Then I carefully parted the vegetation from
top to bottom and from edge to center, till I had
searched the entire volume of vegetation.
For each plot, I recorded the number of webs of
each Argiope species, the height of placement of each
web (distance in cm from the ground to the hub),
the height in cm of the vegetation where each web
was, and the instar1 of each spider. The instar was
estimated from comparison with the size of preserved
laboratory-reared specimens of Argiope aurantia.
Since the instar of A. trifasciata was judged using
specimens of A. aurantia, the estimates for instars of
A. trifasciata were less accurate. When collected
specimens of both species were reexamined in the
laboratory using a dissecting microscope, it was found
that the field and laboratory estimates differed by no
more than one instar.
Data were gathered only after 1000 (to avoid
dew), before 1700 (to avoid heavy highway traffic),
and on sunny days (to help see webs). In August
and September, one and two additional plots, respec-
tively, were searched immediately next to each plot,
because of the low densities of spiders. Statistics
were computed with programs by A. J. Barr and J.
H. Goodnight (Department of Statistics, North Caro-
lina State University, Raleigh) and the facilities of
the Triangle Universities Computing Center.
Incomplete information was gathered for some
webs because of: (1) destruction of the web or
distortion of the vegetation to which the web was
attached, (2) failure to find a spider on the web
(such webs were counted, but the spider’s instar
could not be described), (3) lack of sufficient time
to examine all webs, mainly in May, 1971, because
the spiders were very numerous (on such occasions
I examined only every second or third web). These
deviations from strict randomness are considered
minor.
Correlations of numbers of the Argiope species
In the lespedeza areas, the numbers of individuals
of the two species became significantly positively
1 The first instar is that stage which remains inside the
cocoon after éclosion, while the second instar is that
which emerges from the cocoon and first builds a web
(Kaston 1948). As McCook (1889) noted, araneids can
mature at various unequally-sized late instars.
Early Spring 1974
STRATIFICATION IN ORB-WEB SPIDERS
319
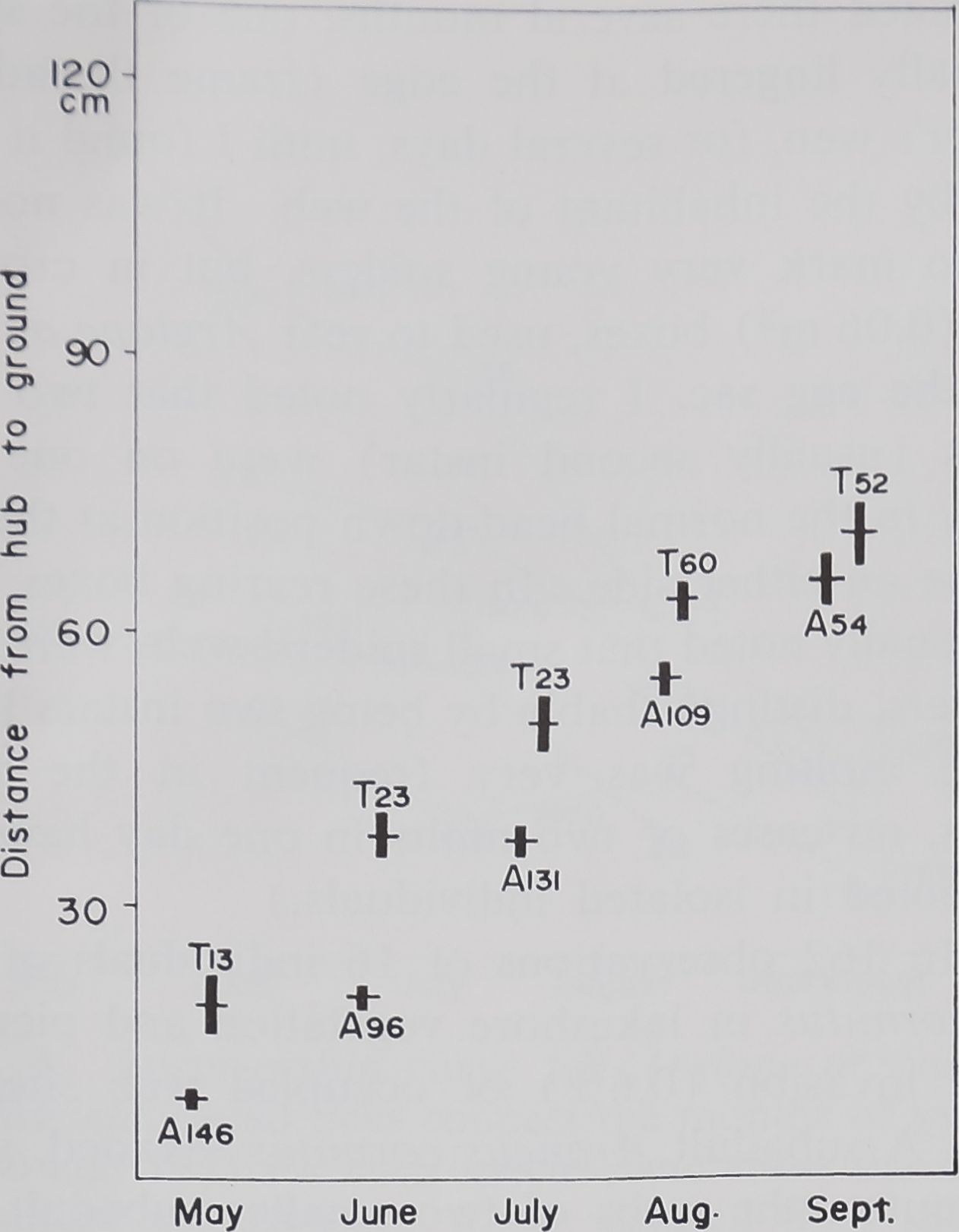
Fig. 1. Height above ground at which
webs of Argiope aurantia (A) and A. tri-
fasciata (T) were located. The barsE: one
SE on either side of the mean, near the bars
are the no. of webs. The ht of location of
webs increases with successive months, espe-
cially by A. aurantia. The two species differ
significantly in the ht of location of webs,
except in Aug.-Sept.
correlated by the end of the summer. Sums of data
for the months of sampling are analyzed in Table 1.
Height at which web was built
Fig. 1 shows the height at which the webs of
Argiope aurantia and A. trifasciata were found in
the random sampling of webs at the end of each
month in 1970 and in May of 1971. For Argiope
aurantia Fig. 2 shows the web heights of the various
instars and height of location of the egg sacs, for
data from the same months. The positive slope of
the data shows that the increase in height in the later
months of the year is related to the increase of instar
from one month to the next. The fact that the lines
which might be drawn connecting data for later
months in Fig. 2 lie ever higher up indicates that
growth of the vegetation during the summer had the
effect of increasing the height at which all instars
situate the web.
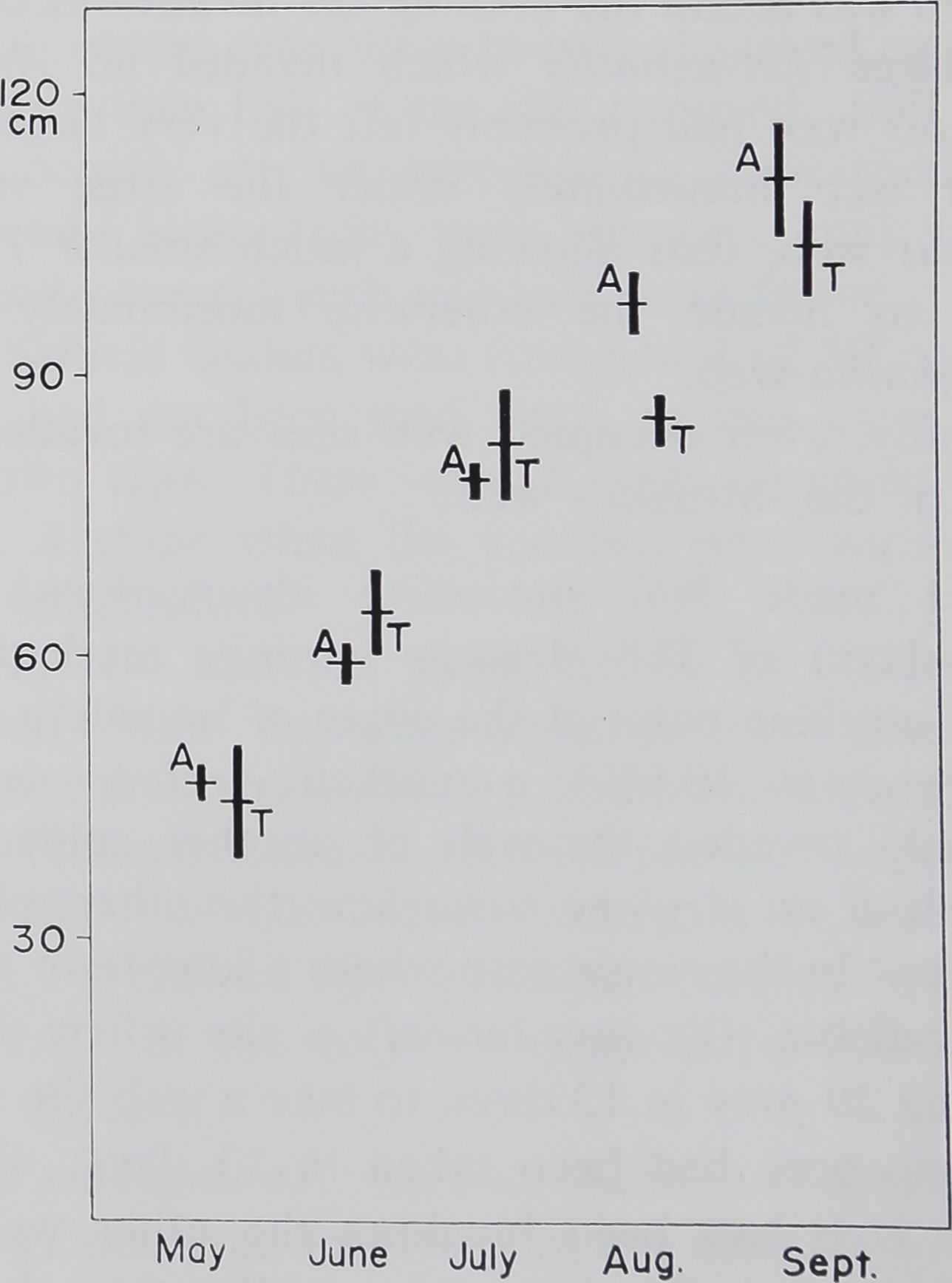
For both species, the height of the vegetation in
which the web is built is shown in Fig. 3. The lack
of a consistent difference between the two species
shows that the difference in the height at which the
webs of the two species are built early in the year
(Fig. 1) is not due to a choice of vegetation that
differs in height. Fig. 1 reveals that A. trifasciata
webs are consistently located higher up than A.
Fig. 2. The dependence of ht above ground on instar
in Argiope aurantia. A solid line connects the data for
the sample taken in July. Numbers are the sample sizes
for instars within each sampling month.
aurantia webs. But, by September, the webs are
no longer at significantly different heights, when
compared by the use of a f-test (Snedecor and Coch-
ran 1967).
Interference among araneids
Independent exploitation is the method of allocat-
ing resources ordinarily assumed. But interference
Fig. 3. Height of the vegetation in which
Argiope aurantia (A) and A. trifasciata (T)
webs were located. The ordinate is the dis-
tance between the top of the vegetation
directly above the web and the ground, in
cm. The bars = one SE on either side of
the mean.
FRANK ENDERS
Ecology, Vol. 55, No. 2
may occur, to prevent the success of the most efficient
competitor (Miller 1967). Web spiders may be
subject to interference by intrusion onto their webs
and preemption of web sites by larger individuals.
Bilsing (1920) recorded cannibalism by Argiope
trifasciata upon individuals of the same species in
less than 1% of 621 webs examined for prey. Late
in the year I also observed a very few cases (less
than 1% of prey) of A. aurantia feeding on A. auran-
tia, A. aurantia on A. trifasciata, and A. trifasciata
on A. aurantia. These cases are females being eaten
by females. Since the prey of araneids ordinarily
must contact the web, this suggests that females of
either species of Argiope will intrude onto the web
of either species.
I have made observations of the invasion of un-
occupied webs: 39 adult female Argiope aurantia
were removed from their webs for an hour between
0220 and 0100 hr, to be weighed; five of the un-
occupied webs were invaded, all by conspecifics. At
a fishing pier, 38 similar cases of removal of Araneus
cornutus Clerck (Araneidae) from their webs resulted
in another five invasions of webs : three conspecifics,
one Eustala sp. (Araneidae), and one Tetragnatha
laboriosa Hentz (Tetragnathidae). Of these intru-
ders, one conspecific was followed and captured off
the web by the original occupant. On later occasions,
I chased the intruder away before returning the
original occupant. The Eustala had a web on the
previous night at the site which it invaded, though
no web was found the evening the invasion occurred.
The large Tetragnatha which invaded an Araneus
cornutus web had probably left the very large hori-
zontal web immediately under the large vertical
Araneus web, thus allowing a much smaller Tetrag-
natha to invade the otherwise inordinately large
Tetragnatha web.
Finally, even occupied web sites are invaded and
used for the intruder’s web:
1) I made 800 successive observations (total
spider-days) of 118 Argiope aurantia marked with
model airplane paint at the edges of lespedeza areas.
In five cases (0.6%) a marked Argiope aurantia
definitely invaded the web of another spider, one
the web of an Argiope trifasciata, the others of con-
specifics. In the single case where a long-term record
was available, the invader left a site where it had
obtained 29 prey in 13 days, to take a web site where
only one prey had been taken in 13 days: the in-
vader’s web had been blocking the other web site
from obtaining the honeybees pollinating the les-
pedeza at that time. In 166 observations of 29
Argiope trifasciata at the edge of lespedeza, only the
one invasion mentioned above was noted.
2) In the laboratory, four Argiope aurantia were
placed in an indoor cage (4 m X 2 m X 2 m), and
maintained there several months; one of the spiders
eventually lingered at the edge (frame threads) of
another’s web, for several days, until I found it being
eaten by the inhabitant of the web. It was not pos-
sible to mark very young spiders, but in crowded,
small (0.06 m1 2 3) boxes, used to rear Argiope aurantia
from the egg sac, I regularly noted that two small
spiders (usually second instar) were on one web,
usually in the normal head-down position at the hub,
but one on either side. In these rearing boxes I also
occasionally noted that small spiders’ webs were taken
by others, distinguishable by being two instars larger.
(While molting was very frequent in the young
spiders, no cases of two molts in one day have ever
been noted in isolated individuals.)
3) In 162 observations of 16 individuals of Ara-
neus cornutus in lakeshore vegetation and pier, one
double invasion (0.6%) of occupied web sites was
noted. A subadult Araneus cornutus invaded, within
15 minutes, the webs of two smaller subadult Neo-
scona arabesca (Walckenaer). This marked Araneus
fprnutus had been present several days, its web closer
to the vegetation than the Neoscona webs and within
15 cm of their vertical faces. In each invasion, the
Araneus climbed rapidly up the Neoscona web from
below, and then from the sides, until it could not find
silk on which to climb. Meanwhile, the Neoscona
ran down from the hub and seemed to cut away the
silk in front of the Araneus, jerking itself back toward
the hub (and the Araneus away) by the release of
the tension of the web each time. In one case, the
invasion began while the Neoscona was handling prey
at the hub of the web, and, in this case, the Araneus
was able to advance further onto the web. That night,
after invading, and somewhat later than the usual
time for building, the Araneus built a very large web
at the site of the Neoscona webs. In 18 observations
of eight Neoscona arabesca, no other web invasions
were noted.
These observations suggest that araneid spiders
may interfere with the use of space by competitors,
by intruding upon one another’s webs. While one
might object that these observations of web invasion
occurred under “crowded conditions,” that is pre-
cisely the point: spiders probably tend to invade
webs, even those of other species, mainly under
“crowded” conditions, so that this behavior must
function as a form of competition for space. I have
not observed that the invaders of webs are under-
nourished, judging from the relative width of abdo-
men and céphalothorax; I have noted, judging from
length of leg or other hard part, that successful
intruders seem to be larger than the original occupant
of the web.
Thus, invasions of occupied webs occurred in 0.6%
of my observations, both in Argiope aurantia and in
Early Spring 1974
STRATIFICATION IN ORB-WEB SPIDERS
321
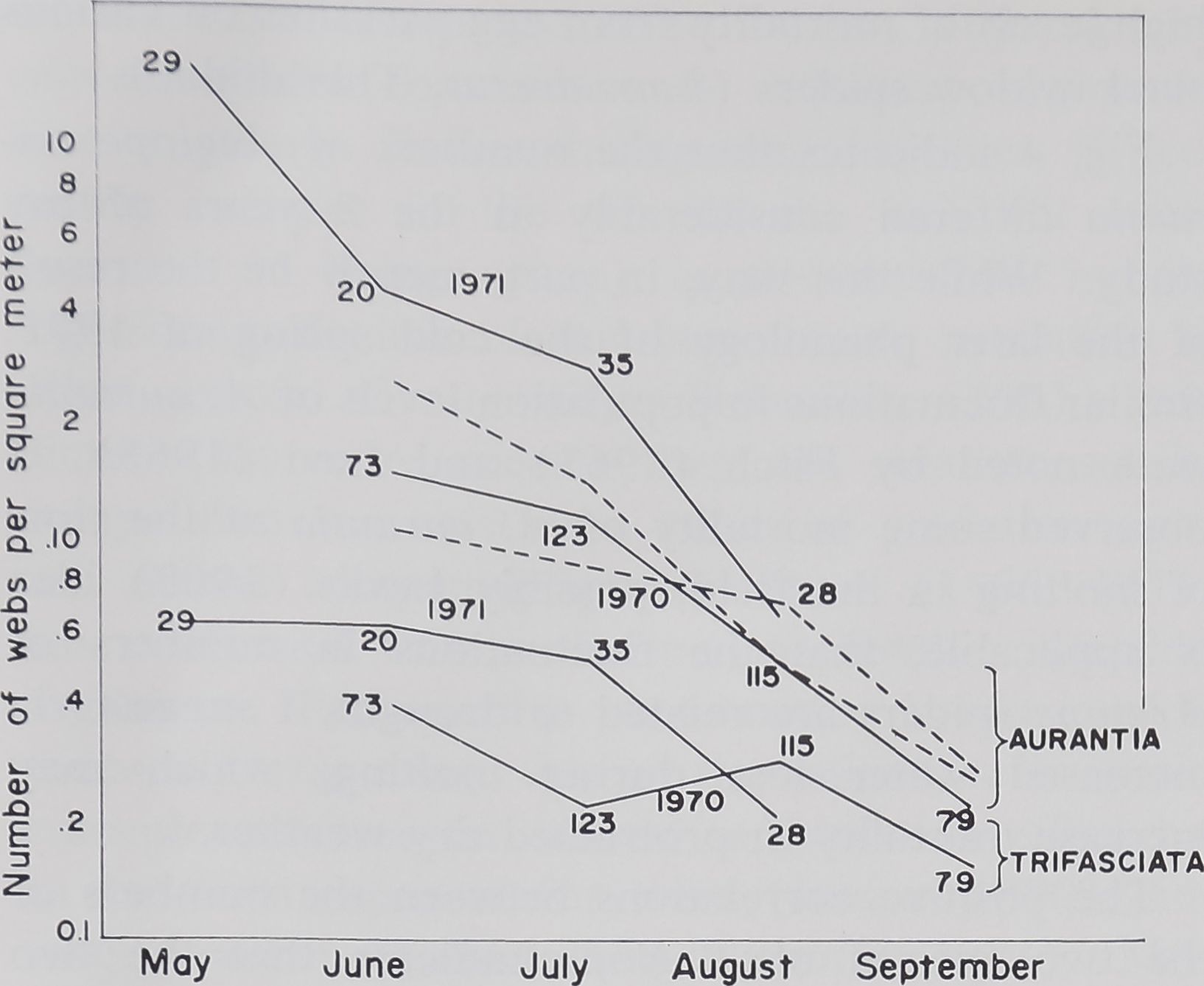
Fig. 4. Survivorship curve for Argiope aurantia and
A. trifasciata. Solid lines connect the months of sampling
in 1970 and 1971 for each species, and show the number
of locations searched for web||j Dashed lines show the
survivorship at the two largest road cuts during 1970
separately for A. aurantia.
Araneus cornutus. This figure is considered a mini-
mum estimate, because if one of the participants in
the invasion was unmarked (and was not two instars
larger) or had been at an unmapped web site, my
methods would not record web invasion. If invasions
occur at a rate of 0.6% per web-day, since each adult
spider has spent about 110 days on an individual web,
most araneids, at least of these species, must have
contended with web invasion during their lives.
Mortality: a partial life table
Fig. 4, illustrating survivorship curves (webs per
square meter) on a semilogarithmic plot, approxi-
mates a straight line, usual for invertebrate animals.
While no marked difference in the rates of mortality
(the slopes of the lines) between the two species is
evident, Argiope trifasciata does appear to have a
slightly lower rate of mortality. The latter could be
an artifact of the later emergence of A. trifasciata
from the egg sacs: the peak numbers of A. trifasciata
on webs may have occurred in early June rather than
late May. Then a steeper, but unobserved, decline
in numbers, as sharp as that for A. aurantia would
have resulted.
The downward curve of the graph of the data for
A. aurantia in September may be due to the dis-
appearance of the (shorter-lived) males by then;
doubling the number of spiders in September elim-
inates the curve, and so provides a better fit to a
straight line.
In 1971, only road cuts 4 and 5 were sampled.
For Argiope aurantia in 1970, data from 4 and 5
(dashed lines) are shown separately. Fig, 4 shows
that the marked increase in numbers of Argiope
aurantia from 1970 to 1971 cannot be due to the
sampling of these areas only. Also, Fig. 4 shows
some compensatory mortality: the increase of
Argiope aurantia from 1970 to 1971 is much reduced
by August.
In 1970 Argiope aurantia from road cut 3 declined
in numbers more precipitately than the other areas.
During observations of marked spiders, more activity
by spider-hunting wasps (Psammocharidae) had been
noted at the edge of that area than in other study
areas. Besides various species of psammocharid
wasps, predators observed attacking Argiope aurantia
(immatures) include Mimetus (probably epeiroides)
spiders, and one Lycos a (probably carolinensis)
spider. Also, the cocoons of Argiope aurantia were
subject to mortality from several predators upon
eggs:
1 ) During August and September several Chaulio-
gnathus beetle larvae (Cantharidae) were collected
from cocoons in which they had partially eaten the
egg masses; most of the cocoons found later in the
year had holes similar to those made by Chaulio-
gnathus’ entrance.
2) In many cocoons the insides were completely
removed, including the silken lining between the
eggs and the outside parchment-like silk. As this
regularly occurred even when the cocoons remained
attached about 90 cm above the ground, this sort of
damage was probably done by birds. Eight of 66
cocoons collected in spring, 1971, when care was
taken to obtain even those heavily damaged egg sacs,
had more than half of the silk removed. Still more
such cocoons can be supposed to have been torn
completely loose from their supports, and so not
recorded—several such egg sacs were found.
3) Salticid spiders were found in 4 of 58 cocoons
which had not been torn open, in two cases, with
their own eggs. These salticids were observed to eat
young Argiope when the cocoons were warmed to
room temperature.
4) In late autumn, 1969, 26.5% of 34 cocoons
collected from lespedeza-covered road cuts near
Raleigh were parasitized by Tromatobia rufopectus
(Cr.) (Ichneumonidae, Hymenoptera). In autumn
1970, 26.0%. pf 100 cocoons collected were so para-
sitized. A chalcid hyperparasite occurred in 44.4%
and 69.2* of the cocoons attacked by Tromatobia
in the 2 years.
5) Pseudogaurax signatus (Lw.) (Chloropidae,
Diptera) occurred in 17.6% of the cocoons in 1969,
and only in 4% in 1970. The cocoons were not
collected on the study areas, though parasitized
cocoons were encountered during September sam-
pling. These last two sources of mortality did not
vary considerably in the 2 years of study: the infesta-
tion of cocoons on the next large road cut to the
322
FRANK ENDERS
Ecology, Vol. 55, No. 2
northeast of road cut 4 (5 faced 4) varied only from
22.7% to 23.8% for Tromatobia, and from 9.1% to
4.8% for Pseudogaurax, of 22 and 21 cocoons col-
lected there from 1969 to 1970. Tromatobia and
Pseudogaurax often left many Argiope aurantia alive
in the cocoons, possibly because they are able to eat
only the eggs. In only one cocoon were both egg
parasites present.
6) Cocoons up to 4 m up the side of the road cuts
were destroyed by annual winter mowing.
I have no information for Argiope trifasciata re-
garding predators, other than the observation of a
web-invading predatory theridiid spider (Rhomphaea
sp.) at the edge of an Argiope trifasciata web. More-
over, I found only four cocoons of A. trifasciata,
none of them damaged; these cocoons were located
considerably lower down than those of A. aurantia.
General Discussion
Muma and Muma (1949) collected Argiope tri-
fasciata on trees and shrubs, but A. aurantia from
herbs. Contrary to their findings, Fitch (1963)
reported that Argiope aurantia usually builds its web
higher up than A. trifasciata. However, this is prob-
ably an artifact of A. trifasciata’s occurring in more
open areas where vegetation is shorter (Fitch 1963,
Enders 1973). In all habitats, therefore, the average
adult A. trifasciata web might be closer to the ground
than the web of the average adult A. aurantia. How-
ever, my data (Fig. 1) show that the immature A.
trifasciata in the habitat studied build webs higher
up than immature A. aurantia. In fact, wherever I
have found both species in the same stand of vegeta-
tion, mainly roadside vegetation and old fields, the
immature A. aurantia built webs closer to the ground,
on the average. I have confirmed this difference in
height chosen experimentally in outdoor cages (En-
ders 1972).
The positive slope of web height of Argiope auran-
tia graphed against instar (Fig. 2) suggests that the
spiders must choose different heights at different
instars. I have evidence that this depends upon
sexual maturity rather than body size (instar) per se.
The cocoons of Argiope trifasciata were generally
lower down and hidden under leaves more than those
of Argiope aurantia. The absence of predation upon
any of the four A. trifasciata egg sacs, contrasted to
the high rate of damage to egg sacs of A. aurantia,
suggests that the location of the former may prevent
birds from seeing them, while the colder weather late
in the year when this species lays its eggs may pre-
clude predation by the arthropods. Wilder (1873)
reported a high infestation of A. aurantia cocoons
with egg parasites, possibly the same as I found.
While an outdoor life table is available for no other
web spider, Abalos and Baez (1967) reported similar
high levels of mortality from egg parasites on various
black widow spiders (Latrodectus, Theridiidae).
Fig. 4 indicates that the numbers of Argiope au-
rantia differed considerably in the 2 years of my
study. While this may, in part, merely be the result
of the later phenology of the cold spring of 1971,
similar fluctuations in population levels of A. aurantia
were noted by Fitch (1963) and Levi (1968). I
observed some mortality of A. aurantia at the time
of molting in the field; possibly Levi’s (1968) idea
is applicable, that the fluctuations in numbers of
Argiope spiders are related to drought, I suggest via
increased water loss during molting, which may
increase mortality in protracted dry weather.
The positive correlations between the numbers of
the two species of Argiope indicate that the two
species are occupying the same horizontal component
of the microhabitat. Niche separation by Cody’s
(1968) scheme must therefore be either by vertical
space or prey items taken.
Turnbull (1964) reported that Achaearanea tepi-
dariorum (Theridiidae), a web-building spider, had
a positive aggregation response to prey abundance.
Enders (1972) found no such response for Argiope
aurantia, and the little data for Argiope trifasciata
also show no aggregation where success at prey cap-
ture is higher. This indicates that the positive cor-
relation between the two species is not due to local
prey abundance; the correlation may be due to the
similar needs of the two species for suitably stout
attachment points for webs and for an open space
between the attachment points of sufficient diameter
for the adult web.
Here I use ecotope, niche and habitat range as
suggested by Whittaker et al. (1973). Late in the
year, the two Argiope species seem to be, in effect,
using the same niche, including food and location
of the web. These species may be able to do so
because they are few, as adults, although, in the case
of an encounter, the larger species (in my experience,
ordinarily A. aurantia) will physically interfere with,
and even eat, the smaller. Argiope trifasciata, as a
species, has a habitat refuge in those stands of
vegetation which are too sparse for the use of A.
aurantia (Enders 1973). Early in the year, these
two species are vertically stratified. Late in the year,
the two species compete directly for web sites, in
most of their ecotopes, due to the changed vertical
distribution of A. aurantia. However, since both
species are becoming fewer, the number of encounters
may be reduced to a tolerable level.
Thus, these spiders are an example of the impor-
tance of both spatial and temporal coincidence for
competitive encounters. Griffiths (1969) pointed up
the importance of such coincidence for predatory
interactions. In general, spatial coincidence must be
less frequent in less mobile organisms, which encoun-
Early Spring 1974
STRATIFICATION IN ORB-WEB SPIDERS
323
ter other individuals less often the less either species
moves; spatial coincidence in sedentary organisms
must regularly decline during their lifetimes, if geo-
metric increase in the size of the web (or other
measure of living space) is overmatched by the
logarithmic decline of numbers due to predation or
other source of mortality. Slobodkin (1961) pre-
dicted that more species than the number set by
competition can exist as a result of predation upon
one of the competing species; Paine (1966, 1971)
has confirmed this prediction for benthic intertidal
organisms. Perhaps this phenomenon is restricted to
sedentary organisms, due to the reduction in spatial
coincidence discussed above: a predation effect has
so far been reported for trees (Janzen 1970), and
resting moths (Ricklefs and O’Rourke 1973). Most
of the animals studied by Paine (1971), and web
spiders also, can be described as sessile filter feeders.
Conceivably, if predation regularly allows species of
invertebrates to coexist, such predation by the verte-
brates may partially explain the great number of
species among such invertebrate groups as the Arthro-
poda.
Evolution of the ecotope of Argiope trifasciata
The situation of the two Argiope species is com-
parable to that studied by Murray (1971) : in differ-
ent geographical areas, two sparrow species dominate
a third, fugitive species (Hutchinson 1951), which
seems to depend for its survival upon the chance
reduction in numbers of the domineering species.
As a result, the subordinate member of the species
pair comes to be adapted to what were originally
“suboptimal” habitats for the genus. For arthropods
(and the intertidal benthos) the chance reduction in
numbers is great in the immature stages and has a
high probability; in fact, for one of the domineering
sparrows, catastrophic mortality due to excessively
high tides has been found in 2 of 4 years of one
study (Post and Enders, unpublished data).
The vertical separation of Argiope trifasciata from
Argiope aurantia can be interpreted both as an evolu-
tionary displacement from the niche of A. aurantia,
and as an adaptation for the use of early stages of
succession: A. trifasciata can colonize cultivated
fields abandoned only 1 year, because it accepts web
sites exposed to more wind, including habitats with
sparser vegetation as well as web sites higher up than
those acceptable to A. aurantia (Enders 1972).
That Argiope trifasciata evolved after A. aurantia
is supported by the fact that, on morphological
grounds, A. trifasciata is a recent offshoot from
Argiope bruennichi of southern Europe (Levi 1968);
the latter seems more like A. aurantia in preferring
a web site near the ground as an immature (Tilquin
1942). The number of mutually allopatric Argiope
species which are sympatric with the cosmopolitan
A. trifasciata in the Americas in different areas (Levi
1968) also supports the recent derivation of A.
trifasciata, and implies that its niche is displaced
from that of the primitive Argiope, which, I suggest,
is a large orb-weaver, near the ground as an immature
but higher up as an adult.
Consideration of the Araneus spiders collected by
Luczak (1963) suggests that Argiope trifasciata must
have evolved to fill the niche of Araneus diadematus
in the New World field-type habitats, in the presence
of Argiope aurantia (which fills the niche of Araneus
quadratus) and Araneus marmoreus. Araneus dia-
dematus has been successfully introduced to the
western hemisphere, but its range here is mainly
restricted to north of that of Argiope trifasciata. This
is as expected—Araneus diadematus in the New
World should occupy a different range of habitats
but the same niche in those colder areas, as the genus
Araneus is probably physiologically adapted to a
cooler climate, Argiope, to a warmer. (Compare the
ranges of the genera, as described by Levi 1968,
1971.)
Coexistence of araneids: specialization for
microhabitat (stratum) or for prey size?
As Bristowe (1958, p. 247) pointed out, there are
two groups of araneid spiders in Europe, spring and
autumn breeders. Within these two groups one
expects to find the species separated by successive
increases of IBM in size, reflecting a doubling of
prey size, and sufficient specialization for food size
only (Hutchinson 1959). Because specialization for
season of breeding also results, in spiders, in distinc-
tion of the spiders’ sizes, the season of breeding may
be taken also to reflect specialization for prey size.
Huczak (1963) collected ten species of Araneus from
stands of young pine trees and heather. Here, I
analyze her data to test: ( 1 ) what fraction of araneid
species depend mainly upon specialization for prey
size for coexistence (via differences in season of
breeding and in size), (2) what fraction of the
araneids show a vertical stratification of species and,
with age, a change of stratum used (specialization for
microhabitat), and (3) whether there is a large
residuum of species apparently coexisting by dif-
ferences along some other, undetermined niche
parameter.
In Table 2, I list for these Araneus species the
length of the adult female spider, the season of
breeding, the increase from the size of the next
smaller species breeding in that season, and the
stratum of vegetation used by immatures (shrub or
heather). In the springtime, one would expect to
find most obvious the differences among species
critical for their coexistence: in spring the spiders
are most abundant, most similar in size (the larger
fall-breeding species present as immatures), and most
FRANK ENDERS
Ecology, Vol. 55, No. 2
Table 2. Ecological differences among the species of Araneus found by Luczak (1963) in stands of heather with
young pine trees. Sizes of adult female spiders taken from Menge (1866) and Locket and Millidge (1953), the
latter the source of data on months of breeding as well. Figures in parentheses are the calculated increase in size
from the next smaller species breeding in the same season; the last columns on the right give the location of the
small spiders taken by Luczak (1963) by beating the vegetation during Aug. and Sept. 1959. Two species of Ara-
neus are excluded because only two specimens of each had been taken by Luczak
Species of Araneus Months of breeding Length of adult female, in mm Average increase in size Location of young collected by Luczak
Locket & Millidge Midpoint Range of range Menge
No. in heather/no. in shrubs Majority in
A. sturmi April-June 3-5 4 4.5 16/31 shrubs
A. cucurbitinus May-July 4-6 5 7 41% 81/124 shrubs
(25%) (56%)
A. redii April-May 5.5-7 6.3 not 25%. 60/14 heather
rmmmm given
A. patagiatus all year 5-8 6 9 9/11 shrubs
A. adiantus July-Sept. 6 6 none/8 shrubs
A. marmoreus Aug.-Sept. 5-8 6.5 13 63%|| 2/4 shrubs
(B— diM
A. diadematus Aug.-Oct. wM 15 37% 16/25 shrubs
(69%) (1—
A. quadratus Aug.-Sept. 12 15 5%l 44/10 heather
(zero)
compressed in their vertical distribution (due to the
presence of only previous years’ growth of vegeta-
tion). Though data for the spring is not available,I
it is certain that the largest autumn-breeding species
will be smaller than the smallest spring-breeder at that
time. I assume that the sizes of the immatures will
be isomorphically related to the sizes of conspecific
adults, as seems to be true for araneids I have worked
with. (Possibly the sexual size dimorphism of spiders
causes males and females to represent two ecological
“species,” males the smaller. Then, one would de-
mand more than 28% difference in size, from Table
2, before one accepts that size is the only significant
niche parameter. According to this view, sexual
dimorphism may explain some of the high values for
difference in size among adult females in Table 2.
But, as young spiders do not show sexual size di-
morphism, the size differences of immatures may
be only 28%; we must remember that the figures in
Table 2 do represent only a first approximation to
their sizes.)
Table 2 suggests that most araneid spiders coexist
by differences related to prey size: season and size
of spider. Most species’ niches are sufficiently dis-
tinguished on the basis of size of spider (and so of
prey) alone. Only one medium-sized species, Ara-
neus patagiatus, breeds throughout the year, and it
also has the most even distribution of numbers of
immatures between the two strata, heather and
shrubs. This species thus seems to be a generalist
which is less efficient at using the limiting resource
(MacArthur and Connell 1966, p. 67), and, as such,
may depend upon the occurrence of unusual mortality
of (any of seven) specialist species to reduce competi-
tion. It must also be able to interfere with the other
species whenever they happen to be smaller than
itself.
Table 2 shows that the smaller spider species breed,
on the average, before larger, except where micro-
habitat differences (shrub or heather used by im-
matures) obviate the need. Thus, in spring, Araneus
sturmi breeds before A. cucurbitinus, and, in autumn,
A. adiantus breeds before A. marmoreus, which is
before A. diadematus. This is contrary to what one
expects from Hutchinson (1959). And, if, as seems
to be true, the young of smaller species are smaller
than the young of larger species, the reason behind
any pattern of phenology is obscure.
The three largest Araneus species, of the autumn-
maturing group, do not show the sufficient differ-
ences in size: Locket and Millidge (1953) show
more than the 28%• size difference between Araneus
marmoreus and A. diadematus, but Menge (1866),
Kaston (1948) and my own experience indicate that
A. marmoreus is smaller than A. diadematus, but not
by the full 28%. But, since Menge (1866), Kaston
(1948) and Locket and Millidge (1953) all indicate
that A. marmoreus uses a retreat in leaves, usually in
damp localities, the coexistence of this species with
the other two large species may be due partly to its
smaller size and partly to the use of a specialized site
for its web.
Luczak (1963) collected large and small specimens
of Araneus by different methods. She states (p. 203)
that in contrast to small specimens of the Araneus
species “the community of large spiders . . . matures-
Early Spring 1974
STRATIFICATION IN ORB-WEB SPIDERS
325
cent and adult forms (A. diadematus Cl., A. quad-
ratus C., A. marmoreus Cl.) is distributed in another
living space, occupying ecological niches between
pine trees.” Luczak’s data on the distribution of the
small spiders, Table 2, shows that 61% of the 41 A.
diadematus were taken from shrubs, but only 18.5%
of the young A. quadratus. Thus the evidence sug-
gests that these two species of Araneus coexist by
the use of different sites for webs while immature.
That 42.5% more of the young A. diadematus use
the shrub layer indicates sufficient specialization for
stratum, as shown by qualitatively measured web
sites in the same way that Reynoldson and Davies
(1970) analyzed kinds of food.
The adults of Araneus diadematus face competition
from the similarly sized Araneus quadratus and
Araneus marmoreus. But, as I have argued above
for Argiope species, the few individuals which survive
to adulthood, and the consequent low number of
possible competitive interactions between the species,
must allow coexistence of the large Araneus species
using the same prey resource in the same stratum of
the vegetation as adults. Araneus quadratus may be
considered an ecological equivalent to Argiope auran-
tia (and Argiope bruennichi), while Araneus dia-
dematus similarly is equivalent to Argiope trifasciata.
Vertical stratification and aggression
While vertical stratification has been reported in
several insectivores, Morse’s work ( 19681 reveals
that much of the vertical stratification of warbler
species (MacArthur 1958) must have been dfc only
to the choice of particular s*dng perches by males.
Andrews (1971) found vertical stratification in
lizards, which also was partly confounded with the
use of certain heights by males when displaying.
Additionally, Handley (1967) has noted vertical
stratification of species of bats. A vertical stratifica-
tion similar to that of the largest orb-weaving spiders
in fields is known to occur between species of the
large widow spiders (Latrodectus, Theridiidae), which
weave three-dimensional webs (Shulov and Weissman
1959; McCrone and Levi 1964; Abalos and Baez
1967). The Latrodectus species actively choose their
characteristic strata (Szlep, 1966), as do the Argiope
(Enders 1972). Richter (1970a, 1970&) has demon-
strated vertical stratification of errant Pardosa
species. Nonetheless, my analysis of Luczak’s (1963)
data suggests that vertical stratification is secondary
in importance to specialization for food size, at least
for spiders. If the animals specializing for stratum
are eating different foods, then vertical stratification
may only be a derived phenomenon, secondary to
food partitioning. E. Waldorf (1973, unpublished
data) finds that, in an evergreen forest herb, different
size classes of spiders are located differentially,
according to the vertical distribution of insect size
classes.
Murray (1971) suggested that horizontal stratifica-
tion of birds, especially of closely related species,
may reduce aggression-eliciting encounters between
look-alike males rather than reduce competition for
food. Edington and Edington (1972) have suggested
that aggression must play a role in spacing within
guilds even distantly related birds. In warblers (Mac-
Arthur 1958, Morse 1968) and lizards (Andrews
1971), vertical stratification also may be the result
of aggression (interference) rather than the result
of exploitive competitionjM
Luczak and Dabrowska-Prot (1970) have observed
cases of inter- and intraspecific invasion of webs of
theridiid spiders, like the invasions of araneid webs
reported here. In neither observations do spiders
show any sense of the “home” web or any knowledge
of the surroundings such as occurs in home ranges of
the vertebrates. Generally, the larger spider is dom-
inant. Spiders thus may engage in interference, like
carnivorous birds (hawks and owls), which have been
reported to devour competitors (Bent 1938, pp. 60,
115, 149, 191, 308, 318) in the course of interfer-
ence, rather than like passerine birds, which engage
in contests of display.
A graded series of invasions of webs is known to
occur, within the family Theridiidae, and again
among the superfamily Epeiroidea (Kaston 1948) of
the order Araneida (spiders) . One family of epeiroid
spiders (Mimetidae) and several species of conopis-
thine theridiids (genus Rhomphaea) make their living
by^Svading webs of other spiders to eat the “host.”
Another group of theridiids (genus Conopistha)
within the Epeiroidea are kleptoparasites which in-
vade the webs of other spiders to eat the prey of the
host. Thus, the occurrence of web invasion during
the lifetime of web building epeiroid spiders, both
theridiid (Luczak and Dabrowska-Prot 1970) and
araneid (this report), may originally represent only
interference by species specialized in size, season, and
stratum. A second step may be the use of physical
interference by a generalist species (such as Araneus
patagiatus or Araneus cornutus, distinguishable by
breeding all year), as a principal method of survival
in a world of specialists. McCook (1889) and Til-
quin (1942) noted an affinity for silk structures by
spiders (“sericophily”). Once the behavior of search-
ing for structures of silk and invading webs was
well-developed, successive arachnophagous and para-
sitic specialist spider species may have evolved from
these generalist species. Thus, competition for space
may occur among web-building spiders, though inter-
ference with webs. This interference seems based
upon similarity of web type, as the vertebrate’s
aggression may be directed by similarity of visual
FRANK ENDERS
Ecology, Vol. 55, No. 2
and aural appearance of the species. In fact, I have
recently found invasion of webs across family lines:
Latrodectus spiders (Theridiidae) having invaded the
webs of Diguetia (Diguetidae), a family of distant
relation, but with similar three dimensional web
structure.
Changes of vertical stratum by insectivores
Changes of the vertical stratum used by an animal
at different ages have not frequently been noted.
Handley’s (1967) data imply that male Anolis poly-
celis lizards must change perch heights as they grow
up. Judging from the data presented by Eberhard
(1971) and Luczak (1963), the web-building spiders
Uloborus diver sus (Uloboridae) and Araneus quad-
ratus may also. I have herein demonstrated that a
change of stratum used occurs in Argiope aurantia.
I recently observed immature and adult Argiope
argentata in different strata. Perhaps any other geo-
graphic replacement of Argiope aurantia, which
serves as the largest orb-weaving spider in a particular
locality, may also change the stratum in which it
places its web. Edgar (1971) and Hallander (1970)
have shown that errant Par dosa spiders (Lycosidae)
change the locality of their search for food from
within the litter layer to above the litter as they
mature. These authors suggest that a change in
food may partly explain the change of stratum with
increase in size.
Why should changes of stratum occur so often in
spiders? Spiders are active predators long before they
are fully mature, and the size of the prey taken is
correlated with the size of the spider. Therefore, ( 1 )
young spiders of larger species are potential com-
petitors with the adults of smaller species, as Hutchin-
son (1959) pointed out for corixid bugs which have
similar life histories, and (2) a change of prey size
must occur as the spider increases in size, assuming
prey size and spider size are correlated. Should the
prey of different sizes occur in different places, a
change of the stratum during the life of the spider
is understandable. In fact, one might expect the
lowest stratum of herbaceous vegetation to have the
most insects, especially of the smaller sizes2 (and so
be a preferred habitat for small spiders, as noted in
the discussion of the evolution of the niche of Argiope
trifasciata), while larger, more active flying insects
occur higher up, even above the mass of the vegeta-
tion. This hypothesized distribution of insects could
explain why smaller Uloborus diver sus (Eberhard
1971) and Argiope aurantia build webs lower than
do larger conspecifics. This reasoning should also
apply to insectivorous reptiles, which change size
2 More small insects may occur near the ground because
individuals from the detritus food chain and those falling
from higher up add to those already on the plant at that
height.
after leaving the parents. During revision of this
manuscript, I discovered that E. Waldorf (unpub-
lished data) studying a woodland perennial herb
supported the ideas developed here: arthropods taken
on sticky traps show a rise in numbers at higher
locations the larger the size of the arthropods. I have
suggested that the significant parameters of smaller
web spiders’ niches may be reduced to prey size alone.
From the data presented above, I conclude that large
web spiders, in addition, use space as a distinct
resource.
MacArthur and Levins (1964) show that searching
animals should specialize for habitat while pursuers
should specialize for the size of prey. Therefore, the
latter occur in arrays of species of different sizes
(Rosenzweig 1966). These theoretical considerations
imply that the smaller species of araneids are acting
as pursuers, since they occur in arrays, while the
larger species of araneids are acting as searchers,
since they specialize in a particular stratum (micro-
habitat) when young. The Par dosa species of wolf
spiders (Richter 1970a and b, Vogel 1972) also
specialize in habitat, and their searching method of
hunting is in line with theory. While the fairly large
Latrodectus species (Theridiidae) also act as searchers
(judging from their vertical stratification), it would
be of interest to know if smaller species of theridiids
occur in sets, as pursuers. That the larger araneids
appear to be searchers for prey may be because they
are so big they take so large a range of prey sizes
that they are unable to expand their food niches vis-
a-vis one another by size differences; instead they
must show spatial segregation.
It is of some interest that the small araneids appear
to be pursuers rather than searchers. Originally, I had
anticipated that the use of webs would place them
either in a separate category from searchers or pur-
suers, or as searchers, like filter-feeders that ought to
take any size prey once extracted from the fluid.
Very tiny prey are regularly ingested by araneids
when the web is eaten prior to renewal. However,
hindsight suggests that capture of prey is a major
struggle for a tiny spider; as the spider becomes larger
a larger proportion of its prey will take relatively less
energy in actual capture, so the spider spends rela-
tively more energy in making contact with some prey.
Thus, a change from a “pursuing” mode of behavior
to one of searching may occur in the lives of filter-
feeding organisms, and, indeed, in the lives of any
organism which retains the same manner of feeding
as it increases in size.
Acknowledgments
This research, in part supported by NSF Grant GB-
6246 to Peter N. Witt, is a portion of a Ph.D. thesis
carried out under his guidance and submitted to North
Carolina State University. During preparation of the
manuscript, the author was supported by NSF Grant GB-
Early Spring 1974
STRATIFICATION IN ORB-WEB SPIDERS
327
27152 to W. F. Blair. K. S. Babu cooperated in rearing
spiders, and R. Pulliam, M. Mares, and E. Yensen made
helpful comments on draft versions of the paper. H. W.
Levi (Museum of Comparative Zoology) has confirmed
the identification of the araneid species, and I must also
thank C. W. Sabrosky and R. W. Carlson (Smithsonian
Institution) and D. Stephens (N. C. State University
Extension) for identifying insects.
Literature Cited
Abalos, J. W., and E. C. Baez. 1967. The spider genus
Latrodectus in Santiago del Estero, Argentina, p. 59-74.
In F. E. Russell and P. R. Saunders [eds.] Animal
toxins. Pergamon Press, Oxford.
Andrews, R. M. 1971. Structural habitat and time
budget of a tropical Anolis lizard. Ecology 52: 26fe
270.
Bent, A. C. 1938. Life histories of North American
birds of prey (part 2), orders Falconiformes and
Strigiformes. U. S. Natl. Mus. Bull. No. 170. 482 p.
Bilsing, S. W. 1920. Quantitative studies in the food
of spiders. Ohio J. Sci. 2Q:wË^260.
Bristowe, W. S. 1958. The world of spideil. Collins,
London. 414 p.
Cody, M. L. 1968. On the methods of resource divi-
sion in grassland bird communities. Am. Nat. 102:
107-147.
Eberhard, W. G. 1971. The ecology of the web of
Uloborus diversus ( Araneae : fflglboridae ). Oecologia
6: 328-342.
Edington, J. M., and M. A. Edington. 1972. Spatial
patterns and habitat partitioning in breeding birds of
an upland wood. J. Anim. Ecol. 41: 331-358.
Edgar, W. D. 1971. The life-cycle, abundance and
seasonal Sjvements of the wolf spideiS Lycosa (Par-
dosa) lugubris, in central Scotland. J. Anim. Ecol. 40:
303-322.
Enders, F. 1972. Web sflj selection by Jmitope aurantia
Lucas and other orb weaving spideB (Araneidae).
Ph.D. Thesis. North Carolina State Univ., Raleigh.
168 p.
——. 1973. Selection of habitat by the spider Argiope
aurantia Lucas (Araneidae). Am. Midi. Nat. 90: 47-
55.
Fitch, H. A. 1963. Spiders of the University of Kansas
Natural History Reservation and Rockefeller Experi-
mental Tract. Mise. Publ. Univ. Kansas Mus. Nat.
Hist. No. 33. 202 p.
Griffiths, K. J. 1969. The importance of coincidence
in the functional and numerical responses of two para-
sites of the European pine sawfly, Neodiprion sertifer.
Can. Entomol. 101: 673-713.
Hallander, H. 1970. Environments of the wolfspiders
Par dosa chelata (O. F. Mueller) and Par dosa pullata
(Clerck). Ekol. Pol. 18: 41-72.
Handley, C. O., Jr. 1967. Bats of the canopy of an
Amazonian forest. Atlas Biota Amazonica 5: 211-215.
Hutchinson, G. E. 1951. Copepodology for the orni-
thologist. Ecology 32: 603-607.
——-. 1959. Homage to Santa Rosalia, or why are
there so many kinds of animals? Am. Nat. 93: 145-
159.
Janzen, D. H. 1970. Herbivores and the number of
tree species in tropical forests. Am. Nat. 104: 501-528.
Kaston, B. J. 1948. Spiders of Connecticut. State Geol.
and Nat. Hist. Surv. Conn. Bull. No. 70. 874 p.
Levi, H. W. 1968. The spider genera Gea and Argiope
in America (Araneae: Araneidae). Bull. Mus. Comp.
Zool. 136: 319-353.
——-. 1971. The diadematus group of the orb-weaver
genus Araneus north of Mexico (Araneae: Araneidae).
Bull. Mus. Comp. Zool. 141: 131-179.
Locket, G. H., and A. G. Millidge. 1953. British
spiders. Vol. 2. Ray Society, London.
Luczak, J. 1963. Differences in the structure of com-
munities of web spiders in one type of environment
(young pine forest). Ekol. Pol. 11: 159-221.
Luczak, J., and E. Dabrowska-Prot, 1970. Preliminary
observations on the food of the spider Theridion pictum
(Walck.) and its predators. Bull. Brit. Arach. Soc. 1:
109-111.
MacArthur, R. H. 1958. Population ecology of some
warblers of northeastern coniferous forests. Ecology
39: 599-610.
MacArthur, R. H., and R. Levins. 1964. Competition,
habitat selection, and character displacement in a
patchy environment. Proc. Nat. Acad. Sci. 51: 1207-
1210.
MacArthur, R. H., and J. H. Connell. 1966. The
biology of populations. Wiley, New York. 200 p.
McCook, H. C. 1889. American spiders and their
ll^Shing work. Vol. 2. Publ. by author and Acad.
~|Jat. Sci., Philadelphia.
McCrone, J. D., and H. W. Levi. 1964. North Amer-
ican spiders of the Latrodectus curacaviensis group
(Araneae, Theridiidae). Psyche 71: 12-21.
Benge, A. 1866. Preussische Spinnen. A. W. Kafe-
mann, Danzig. 560 p.
Miller, R. H. 1967. Pattern and process in competi-
tion. Adv. Ecol. Res. 4: 1-74.
Morse, D. H. 1968. A quantitative study of foraging
of male and female spruce-woods warblers. Ecology
49: 779-784.
Muma, M. H., and K. E. Muma. 1949. Studies on a
population of pra|gfe spiders. Ecology 30: 485-503.
Murray, B. G., Jr. 1971. The ecological consequences
of interspecific territorial behavior in birds. Ecology
52: 414-423.
Mpl R. TV 1966. :^Hood web complexity and species
diversity. Am. Nat. 100: 65W75.
• 1971. A short-term experimental investigation
of resource partitioning in a New Zealand rocky inter-
tidal habitat. Ecology 52: 1096-1106.
Reynoldson, T. B., and R. W. Davies. 1970. Food
niche and coexistence in lake-dwelling triclads. J.
Anim. Ecol. 39: 599-617.
Richter, C. J. J. 1970a. Relation between habitat struc-
ture and development of the glandulae ampullaceae in
eight wolf spider species {Pardosa, Araneae, Lycosidae).
Oecologia 5: 185-199.
——-. 19706. Aerial dispersal in relation to habitat
structure in eight wolf spiders species {Pardosa, Ara-
neae, Lycosidae). Oecologia 5: 200-214.
Ricklefs, R. E., and K. O’Rourke. 1973. Aspect diver-
sity in moths: a temperate-tropical comparison. Sci-
ence (in press).
Rosenzweig, M. L. 1966. Community structure in sym-
patric carnivores. J. Mammal. 47: 602-610.
Shulov, A., and A. Weissmann. 1959. Notes on the life
history and potency of venom of the three Latrodectus
species of Israel. Ecology 40: 515-518.
Slobodkin, L. B. 1961. Growth and regulation of ani-
mal populations. Holt, Rinehart and Winston, New
York. 184 p.
Snedecor, G. W., and W. G. Cochran. 1967. Statistical
methods. Iowa State Univ., Ames, Iowa. 593 p.
Szlep, R. 1966. The web structure of Latrodectus
328
FRANK ENDERS
variolus Walck. and Lcitrodectus bishopi Kaston. Israel
J. Zool. 15: 89-94.
Tilquin, A. 1942. La toile géométrique des araignées.
Presses Universitaires de France, Paris. 536 p.
Turnbull, A. L. 1964. The search for prey by a web-
building spider Achaearanea tepidariorum (C. L. Koch)
(Araneae, Theridiidae). Can. Entomol. 96: 568-579.
Vogel, B. R. 1972. Sympatric occurrence of some
Ecology, Vol. 55, No. 2
Pardosa species (Araneida: Lycosidae). Armadillo
Papers No. 6. 12 p.
Whittaker, R. H., S. A. Levin, and R. B. Root. 1973.
Niche, habitat, and ecotope. Am. Nat. 107: 321-338.
Wilder, B. G. 1873. The habits and parasites of Epeira
(Argiope) reparia, with a note on the moulting of
Nephila plumipes. Proc. Am. Assoc. Adv. Sci. 22:
257-263.