Images Collection
Read OCR Digitized Article Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
Anim. Behav1979, 27,157-164
WEB-SIGNAL PROCESSING FOR TOLERANCE AND GROUP PREDATION IN THE SOCIAL SPIDER MALLOS GREG ALIS SIMON
By J. WESLEY BURGESS North Carolina Division of Mental Health Services, Research Section, P,0. Box 7532, Raleigh, North
Carolina 27611
Abstract. Most spiders are aggressive and cannibalistic but Mallos gregalis lives in large, tolerant colonies where prey is caught communally. Tolerance and communal predation can be attributed to vibration transmitting characteristics of the communally built sheet-web. Sine wave vibrations on the web are attenuated below 30 Hz and above 700 Hz. Within this range, resonance peaks correlate with communal predation behaviour. The web enhances fly vibrations at frequencies in the optimal vibration sensitivity range of spiders. This modulated vibration forms the communal predation cue, co-ordinating concurrent behaviour of many individuals. Conspecifics do not elicit communal predation because their walking vibrations are attenuated. This tolerance mechanism and system for discriminating prey from conspecifics makes a social lifestyle possible for these predatory animals.
The formation and continuation of social groups that contain hundreds or thousands of interdependent members is a difficult lask from the standpoint of behaviour. Unlike group-foraging species such as social ants and bees, herd ungulates, or colonial rodents, spiders are preadapted by the morphology of their mouthparts to a predatory lifestyle. Like wolves or social cats they face the dual problem of organizing group predation and avoiding the problems of aggression or, in the case of most spiders, cannibalism. In contrast to tolerance mechanisms of higher animals, which often depend on agonistic displays and vocalizations of great complexity, spider communication is simpler and mainly vibrational (Witt 1975).
The Animals
Mallos gregalis Simon (— Coenothele gregalis) is a Mexican species of social spider that builds colonies containing thousands of members (Diguet 1915; Millot 1949; Gertsch 1949; Burgess 1976). Both laboratory and natural colonies consist of a continuous swath of webbing enwrapping whole twigs and branches. The web is built communally and has an adhesive surface sheet-web for prey-catching and complicated silken tunnels and walkways in the interior, leading to chambers where egg-sacs are placed and spiders may rest (Burgess & Witt 1976). The usual prey, houseflies and blowflies, are larger (about 20 to 30 mg) than the biggest adult female spider (2 to 10 mg), and predation is usually successful through the co-ordinated efforts of many spiders. When a fly is ensnared on the adhesive surface-sheet, it begins a stereotyped buzzing. At that moment as many
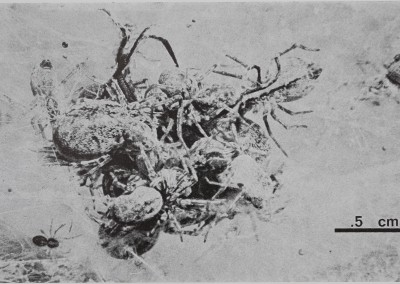
as 30 spiders come running out of the interior and over the surface of the web. They overpower the fly without wrapping it in silk or using the long paralysing bites characteristic of predation by most other species (Plate II, Fig. 1). As the first spiders bite the prey, it struggles and buzzes even more violently than before. When the fly is subdued, small immatures and tiny spiderlings that have not taken part in catching are allowed to feed on the partially predigested prey. Occasionally, flies are transported across the surface of the web and into the interior of the colony, where they may be shared or stored. At no time in the energetic predation sequence do spiders show aggression toward one another, nor is any interspider predation ever seen.
Communication Mechanisms: Tolerance and Prey Discrimination
Most solitary spiders are aggressive and cannibalistic (Bristowe 1958) and do not discriminate between prey and conspecifics. Some active behaviour that probably discourages cannibalism has been found in the Mexican cactus colonies of Metepeira spinipes (Burgess & Witt 1976), the connected aggregations of webs of Cyrtophora citricola (Blanke 1972), the Costa Rican stream-side colonies of Metabus gravidus (Buskirk 1975), the aggregations of the tiny tent-building Oecobius civitas (Burgess 1976), and the commensal tangles of Physo-cyclus dugesi (Burgess 1978). These spiders form aggregations of adults in interconnecting individual webs. Shaking, leg jerking, and retreat are typical consequences of spider interactions, and cannibalism between colony members is uncommon. In some maternal species, female
157
158
ANIMAL BEHAVIOUR, 27, 1
spiders (Nielsen 1932; Tretzel 1961; Kaston 1965; Kullmann 1968, 1972; Rovneretal. 1973) maintain close contact with their offspring without preying on them. Rovner et al. have demonstrated tactile recognition of the mother mediated by specialized abdominal hairs in the wolf spider, Lycosa punctulata. In groupfeeding spiders where adults share a communally built web such as M. gregalis (Burgess 1976, 1978), Agelena consociata and Agelena repub-licana of Africa (Krafft 1970), Anelosimus eximius in subtropical North America (Kaston 1965; Brach 1975), and Stegodyphus sarasin-orum from Asia (Kullmann 1972; Jacson & Joseph 1973), adults commonly confront and touch each other with their palps and legs but do not attack. Krafft (1974) has shown that short-range behaviour (touching) prevents A. consociata from biting each other. However, Krafft (1974: studying A. consociata), Kullmann & Zimmerman (1971, studying S. sarasinorum), and F. Vollrath (personal communication on A. eximius) present evidence that non-discrete vibration can elicit orientation and other preliminary predation behaviour from colony members at long range. An obvious advantage in avoiding aggression, interference, and energy waste comes with distinguishing at a distance between prey and fellow spiders, as in M. gregalis.
Information Transfer: Vibration
This paper investigates the co-ordination of hunting and fly-predation and the prevention of interspider cannibalism and aggression by means of vibration signals (Burgess 1975). M. gregalis has poor vision, but vibration along silk threads seems to be the primary mode of information transfer among web-building spiders (Witt 1975) and is reported to be an important preadaptation to sociality (Shear 1970). This is fortunate for the investigator, because vibration signals are readily detected and analysed. If a web thread running between two spiders is monitoredgt is possible to determine information flow between them (Burgess & Witt 1976).
Vibration signals on solitary webs have been the object of much study (see review in Witt 1975; Barrows 1915; Baltzer 1923; Chrysanthus 1935; Walcott & van der Kloot 1959; Liesenfeld 1961; Bays 1962; Szlep 1964; Parry 1965; Barth 1973). Although sound-pressure responses are known (Walcott 1963, 1969; Frings & Frings 1966), observations suggest that they are not a major factor in prey capture by M. gregalis. One
proposed vibration receptor in spiders is the lyri-form organ that Walcott & van der Kloot (1959) studied in Achaeranea tepidariorum. Although not identical morphologically (Plate III, Fig. 2), lyriform organs are present in M. gregalis and may serve the same purpose. When a spider anchors its leg on a web, the combination of silk, leg, and receptor is all part of the signal processing and transmitting network. M. gregalis webs are well adapted to carry vibration information (Burgess & Witt 1976), being composed of thick silk lines with few weak interconnections in contrast to other webs (e.g. Parry 1965), which show severe overall signal-damping. In physiological studies, Walcott (1969) found uniform, frequency-independent lyriform-organ-receptor response with the greatest sensitivity concentrated between 80 and 800 Hz. It would not be necessary to have frequency-tuned receptors, however, if frequency processing of the signal occurred in the web.
General Methods
Subjects
M. gregalis breed prolifically in a climate-controlled laboratory, and colonies like those in nature containing hundreds or thousands of members are easy to rear. Laboratory colonies were started with the adults and eggsacs that Hcollected from Guadalajara, Mexico, and at the time of this study all individuals in these colonies were presumably from laboratory-born generations. The two colonies each contained about 500 individuals including females, immatures, spider-lings and a few males. Spiders were fed from cultures of Musca domestica (houseflies), at intervals of about a week. Water was provided at feeding time by spraying the web with a plant mister. Colonies were housed in custom-built wooden cages about 32 x 32 X 32 cm, provided with three copper-screen walls and a glass top for observation. The third wooden wall detached as a door for experimental access.
Apparatus and Procedure
Web-borne vibrations were recorded with a high-compliance magnetic transducer (Pickering P/AC-1) held in a micromanipulator. The tip of the transducer was placed on a fragment of thin cover glass 2 to 3 mm long, which was fixed to a web strand by the naturally adhesive cribellate silk. The pickup could resolve both transverse and longitudinal vibration waves, and since no difference was found, the two signals were combined. The signals recorded from the web
PLATE II

Fig. 1. Communal predation in a colony of the spider M. gregalis. Several spiders of different ages combine efforts in subduing and eating a fly that landed in their web and is now covered by the predators. Note small juvenile spider at left of cluster and larger female at right top. Photo by M. Scarboro.
Burgess, Anim. Behav., 27, 1
BURGESS: SOCIAL SPIDER PREDATION SIGNALS IN MALLOS GREGALIS
ANIMAL, BEHAVIOUR 27, i
PLATE III

Fig. 2. SEM photomicrograph from the ventral surface of the tibia on the front leg of an adult male M Note the resemblance to the lyriform organ (Walcott & van der Kloot 1959), a proposed web vibration recento^^f t by L. Jackson. p r‘ Ptloto
Burgess, Anim. Behav., 27, 1
BURGESS: SOCIAL SPIDER PREDATION SIGNALS IN MALLOS G REG ALIS
159
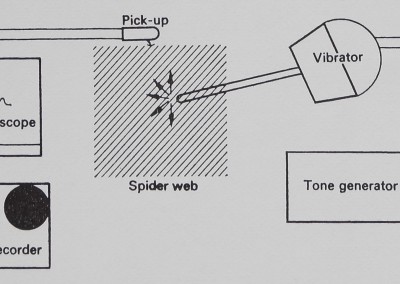
were fed directly into an oscilloscope equipped with a camera, and could also be recorded on a tape deck (Tandberg 6000-X) through the low-level input (see Fig. 3).
Vibration was added to the web from a vibration transducer (Altec) driven by a tone generator (Krohn-Hite Model 5100) with 15*5-VAC sine waves (Fig. 3). The vibration transducer was fitted with a 13*5 cm custom-machined aluminium rod. The vibration transducer assembly had one narrow resonance peak at 90 Hz indicated in the figures. All equipment was calibrated with a Mechanical Technology Inc. Model KD-45A photo-optic transducer. (Since the MTI equipment records displacement optically without touching the vibrating surface, it is perfect for spider-web vibration measurement and is being adapted for that use. Future investigations will be conducted wholly with optic-sensor equipment.) Tape-recorded signals from webs were analysed on a Brüel & Kjaer Frequency Analyser (Type 2107) and a Brüel & Kjaer Level Recorder (Type 2305).
Experiment Is The Vibratory Nature of the Communal Predation Cue
The first experiment is a compilation of observations on how spiders respond to different types of prey items and illustrates an all-or-none response to 10 to 50 of each prey type presented over a six-month period. Non-buzzing prey items observed were (1) houseflies (Musca domestica) anaesthetized with CO 2, (2) houseflies with immobilized wings, and (3) small and large cockroaches (Blattela germanica and Periplaneta americanus). Normal flies that buzzed steadily, those that buzzed intermittently, or
those that escaped from the web after buzzing were also observed.
All prey animals crawled through the threads of the catching sheet-web until they became entangled or escaped. When they became entangled, non-buzzing prey items struggled violently as their legs, wings, and body parts adhered to the adhesive silk. Normal flies also became entangled, and in addition to struggling they rapidly fanned their wings (and possibly halteres) for many minutes or until preyed upon.
Two types of spider responses to the prey items were seen, depending on whether prey buzzed or not. Non-buzzing prey were largely ignored by the colony. If a single M. gregalis touched or was near such prey, the spider might approach and feed individually. No group predation was observed for any non-buzzing prey items.
In contrast, flies that buzzed on the web quickly attracted from 4 to 10 M. gregalis in communal predation. Predatory behaviour characteristically involved groups of spiders coming out on the surface of the web, orientating in jerky jumps, and running in straight lines toward the prey. If the buzzing stopped for a moment, or the fly escaped, all spiders stopped where they were and became very active again only if buzzing resumed. All these observations point to a vibration signal produced by the stereo-typically buzzing fly. The remainder of this study is an investigation of the role of vibration as a cue for communal predation behaviour.
Experiment 2: Comparison of Fly Vibration On and Off Web
Houseflies (Musca domestica) were placed on a laboratory web of M. gregalis, where they began

Fig. 3. Vibration testing apparatus. Vibrations travelling on the sheet-web were received by the magnetic pick-up and displayed on the screen of an oscilloscope, where they could be photographed. The same signals were connected to the inputs of a tape recorder, where they were stored on magnetic tape for further analysis. Vibrations could be introduced onto the web from a vibrator connected to a tone generator.
160
ANIMAL BEHAVIOUR, 27, 1
stereotyped buzzing behaviour. The web vibration was picked up by a magnetic transducer 5 to 10 cm away and was recorded on magnetic tape. Similar recordings were made from flies from the same rearing population that were placed on sticky tape attached to the transducer or grasped firmly by the leg with fine forceps (which also elicits buzzing) and held directly against the transducer. Numerous recordings of both types were made and then analysed on the audio frequency analyser.
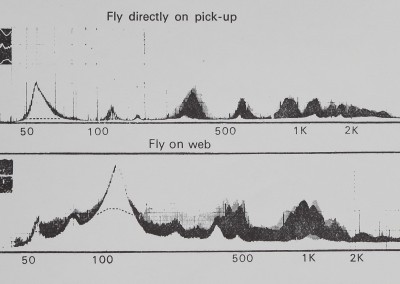
Two representative B & K audio frequency spectrograms are shown in Fig. 4. Differences among individual spectrograms are primarily in amplitude and secondarily in slight displacement of the curves; the basic peaks shown are not different. The insets illustrate photos taken from the oscilloscope screen during the fly vibration. Dotted lines illustrate the possible presence of a 60-cycle artifact. The two curves are considerably different beyond background noise or variability. The direct vibration of the fly shows appreciable higher-frequency (< 1000 Hz) peaks, while the oscilloscope trace indicates
high-frequency vibration with variable amplitude. The amplitude-frequency plot made with the sheet web between fly and pickup has different peaks and a greater concentration of lower-frequency energy.
Experiment 3: Effect of Sheet Web on Vibration Transmission
Since the prey-catching sheet-web of M. gregalis appeared to have an effect on input signal frequency and amplitude, an investigation was performed to determine the nature of this signal change. To do this, a flat-response, sine-wave signal from a tone generator was transduced into vibration and fed onto the surface of the web. The signal was maintained at the same amplitude while the frequency band was swept from 10 Hz to 100 000 Hz. This vibration travelled through the web and was received by the magnetic pick-up 5 to 10 cm away. The resulting amplitude of each frequency signal was measured directly from the oscilloscope screen. Locations were chosen for testing at random over the surface sheet-webs of two M.
Fig. 4. Two representative amplitude frequency plots produced by analysing recorded tape loops of fly buzzing vibration on a B & K Frequency Analyzer and Level Recorder. The abscissa is linear scaled amplitude and the ordinate is a logarithmic scale of frequency. The top curve illustrates the vibration of a fly buzzing directly on the transducer pickup. The lower curve illustrates the effect of intervening sheet-web on the vibration of a buzzing fly. The inspts are photographs taken directly from the oscilloscope screen of the respective fly vibrations. The difference between the two curves shows how the fly vibration spectrum was altered in frequency by passing through the sheet-web.

BURGESS: SOCIAL SPIDER PREDATION SIGNALS IN MALLOS G REG ALIS
161
gregalis laboratory colonies. A mean of results from 12 trials, each generating more than 35 readable data points, is shown in Fig. 5.
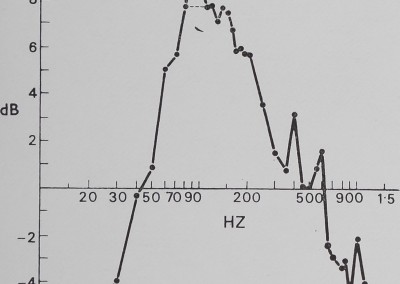
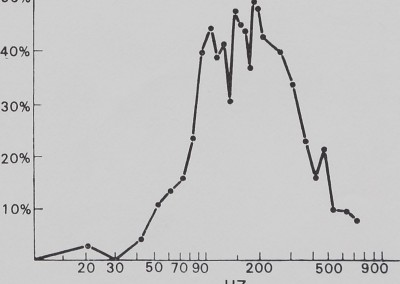
The catching sheet-web of M. gregalis is not a flat vibration conductor. There is significant attentuation of signal amplitude from antiresonance (i.e. signal damping) below 30 Hz and above 700 Hz. This effectively restricts strong signals to the 40 to 600 Hz frequency interval. Within that interval, there are resonance windows (where incoming vibrations are enhanced) approximately between 50 and 200 Hz, between 350 and 450 Hz, and between 500 and 600 Hz. The dotted line shows a possible artifact of vibrator resonance. These windows correspond roughly with peaks illustrated in Fig. 4 (fly on web).
Experiment 4: Effect of Vibration Frequency on Communal Predation Behaviour
In order to determine whether vibration frequency acts as the cue for communal predation, the following experiment was performed. Signal sweeps of all frequencies from 20 to 10 000 Hz at equal amplitude were fed into the web as in experiment 2. Instead of monitoring output vibration, however, the spiders in the web were observed for communal predation behaviour. Before each trial, sample groups were selected averaging nine spiders each that were inside the web and motionless. The criteria for

Fig. 5. The effect of the sheet web on pure vibration. Sine wave vibration across the frequency band was fed into the sheet web and recorded by the magnetic pickup. Below 30 Hz and above 700 Hz, significant attenuation is apparent, while within this band resonance peaks can be seen. This is evidence that the sheet web selectively alters vibration frequencies.
scoring communal predation response were as follows: when vibration was applied the spider must move toward the surface of the web, it must demonstrate jerky turning movements that may be accompanied by a run to the source and, when the vibration is turned off, the spider must again become immobile. The percentage of observed spiders meeting criteria is used as a measure of predatory response to each vibration cue presented. Nine trial sets were run at different sites over the web, and 29 frequencies were tested between 20 and 700 Hz. Spiders were given 30 s to respond to each tone and then allowed 1 min to recover.
The communal predation behaviour of the spiders was predictable by vibration frequency (Fig. 6). No significant response occurred below 40 Hz, above 700 Hz, or in the small 500 to 600 Hz window found in the web response curve (Fig. 6). Most of the response was concentrated between 100 and 400 Hz, which corresponds well to the main resonance described in experiment 2. Coefficient of correlation tests between web response to vibration and behavioural response to vibration show a significant correlation of 0*80 (P < 0-001, t = 6-8, N — 28). Between 20 and 700 Hz, 65 % of the variation of communal predation behaviour is explained by web response (correlation coefficient2 X 100 == % variation).
Discussion
Non-buzzing prey items (cockroaches and experimentally modified houseflies) failed to elicit

Fig. 6. The effect of sheet web-modulated vibration frequency on communal predation behaviour. Sine wave vibration across the frequency band was fed into the sheet web and spiders were observed for predation behaviour. There is a strong, significant correlation between the percentage of predation behaviour and the resonance characteristics of vibration processing in the sheet-web (compare Fig. 5).
162
ANIMAL BEHAVIOUR, 27, 1
communal predation, although these items were sometimes eaten by single spiders. Flies that buzzed on the web elicited a full sequence of predatory behaviour simultaneously from many spiders.
In contrast to recordings made from a solitary-spider web of A. tepidariorum (Walcott 1963, 1969), signals from flies ensnared in a M. gregalis web are altered by passing through the web. Some signals are enhanced and others attenuated, depending on their frequency. Signal energy is concentrated in the frequency range where the lyriform organs of other species have been shown most sensitive (Walcott 1969). Vibrations on the web below 30 Hz and above 700 Hz are subject to attenuation that easily would render them below the threshold sensij tivity of the spiders, while weak, low-amplitude signals could be made more perceptible if they occurred in one of the resonance windows. Thus, no matter which vibration features carry salient information to the spiders (amplitude coding, frequency, phase modulation, etc.), the frequency must correspond to the resonant ranges in order for the signal to be transmitted effectively.
The resonance characteristics of the web explain the elicitation of communal predation behaviour and can be thought of as processing vibration input to cue concurrent response of many spiders to the prey. The vibration need only be responsible for the long-range behaviours: drawing groups from inside the web, releasing jerky orientation turns, and stimulating simultaneous running to the source of vibration. At close range, after spiders reach their prey, tactile cues may also come into play to mediate biting. Because walking spiders cannot produce cue vibrations that elicit communal predatory behaviour, there is no recruitment, orientation, or group predation directed toward fellow colony members.
Walcott (1963) has pointed out that the main concentration of energy in the vibration of a housefly is below 1000 Hz, while the vibration of a honeybee is mainly above 1000 Hz. Muscid flies represent the main diet observed in nature for M. gregalis, while bees and other hymen-optera are not usual prey, presumably because of their sting. Since there would be no selection for response to non-prey items, web attenuation properties may also provide a mechanism blocking approach to ensnared bees.
Conclusions
The web of M. gregalis acts as a signal processor to selectively change prey vibration before it reaches the spiders. The amplification of the signal occurs at the frequencies that are prominent in the vibration spectrum of a stereo-typically buzzing fly and in the presumed sensitivity range of the spider. In this sense, the web tailors information from fly prey so that it can be transmitted most efficiently by the web and received by the spiders.
The success of communal predation stems from the spiders’ ability to locate and converge on the correct prey. The vibratory predation cue not only selects the vibrations characteristic of flies, but also co-ordinates the timing of predation, since flies buzz most predictably when they are already entangled in the web or when they have initially been bitten by a single spider (Burgess 1976). Web resonance provides a high amplitude signal identifying correct prey over a large web area and may attenuate signal information from less desirable prey that have a higher vibration frequency (bees) or lower frequency (cockroaches and hard-bodied beetles). Certainly there would be no selection for response to vibrations at non-prey frequencies. Since spiders normally do not produce the cue frequencies, they are not recognized as prey.
M. gregalis does not bind struggling prey with silk or employ long paralysing bites like many solitary species. The absence of these predatory strategies is compensated for by the high success of co-ordinated group predation. Through group predation, M. gregalis individuals can utilize prey much larger than themselves. The advantages of the best predators (the strongest and fastest spiders) against struggling prey are shared by very young and heavy, gravid female spiders, which may not have participated in prey hunting. The lack of silk-binding, specialized bites and a generalized predatory cue decreases the likelihood of aggression or predation between members. When animals can live together peacefully in a large group they can reap benefits of social living such as increased shelter, better chances of finding a mate, greater area of the catching web, more food available for young and so forth, which are not available to individuals of more competitive, cannibalistic species. In short, M. gregalis colonies represent a trade-off between social concessions and mutai benefits that form an interdependent, self-perpetuating colonial system.
BURGESS: SOCIAL SPIDER PREDATION SIGNALS IN MALLOS G REG ALIS
163
Vibration signals in M. gregalis allow capture of large quantities of food by co-ordinated efforts of group members and preserve tolerance in the colony. Communal predation without tolerance would probably not represent an Evolutionarily Stable Strategy (see Maynard Smith 1976). Individuals in colonies with high rates of cannibalism would run a greater risk of being eaten and reap fewer benefits than individuals in tolerant colonies with communal behaviour. At least two stable predation strategies are seen in the genus Mallos : where vibration discrimination leads to tolerance in M. gregalis, living, web building, prey catching, and feeding take place together. In M. dugesi, M. niveus (R. Jackson, personal communication), and other non-tolerant species, web building, prey catching, and feeding are all confined to separate individuals.
Since other spiders in the Mallos genus may live in fortuitous aggregations but show neither group tolerance nor communal predation (Chamberlin & Gertsch 1958), the use of signals processed by the web may be the shortest evolutionary step from these species to large co-ordinated colonies like M. gregalis. Because not all the resonance windows in the M. gregalis web have been seen to be used in predation, other vibration information (such as mating communication) could be sought there. In the future, investigations of information transfer systems like this one offer another means of understanding behavioural dynamics in social groups.
Acknowledgments Sincere appreciation must be acknowledged to Dr Peter Witt for support and suggestions, to Dr Gilbert Gottlieb for generously allowing the use of frequency analyser and other acoustic equipment, to Dr Willis Gertsch for kindly identifying species, to Dr David Miller for manuscript suggestions, to Mr Larry Gardner of AudioFonics, Inc. for help with acoustical questions, and to N. C. State University, Raleigh, North Carolina. Support was provided by NSF grant GB 25274.
REFERENCES
Baltzer, R. 1923. Beiträge zur Sinnesphysiologie und Psychology der Webspinnen. Mit. Nat. Ges., Bern, 163-187.
Barrows, W. M. 1915. The reactions of an orb-weaving spider, Epiera sclopetaria Cl. to rhythmic vibrations of its web. Biol. Bull., 29, 316-332.
Barth, F. G. 1973. Bauprinzipien adäquater Reize bei einem Mechanoreceptor. Verh. Dtsch. Zool. Ges., 66, 25-30.
Bays, S. M. 1962. A study of the training possibilities of Araneus diadematus. Cl. Experientia, Basel, 18, 423.
Blanke, R. 1972. Untersuchungen zur Oekophysiologie und Oekethologie von Cyrtophora citricola, Forskal in Anadalusien. Forma et Functio, 5, 125-206.
Brach, V. 1975. The biology of the social spider Analosi-mus eximius (Araneae: Theridiidae). Bull. S. Cal. Acad. Sei., 74, 37-41.
Bristowe, W. S. 1958. The World of Spiders. London: Collins.
Burgess, J. W. 1975. The sheet web as a transducer, modifying vibration signals in social spider colonies of Mallos gregalis. Neuroscience Abstracts, 1, 557.
Burgess, J. W. 1976. Social spiders. Scient. Am., 234(3), 100-106.
Burgess, J. W. 1978. Social behaviour in group-living spider species. Arachnology Symp. Zool. Soc., London. (Ed. by P. Merrett) pp. 69-78. London: Academic Press.
Burgess, J. W. & Witt, P. N. 1976. Spider webs: design and engineering. Interdiscip. Sei. Reviews, 1, 322-335.
Buskirk, R. E. 1975. Aggressive display and orb defense in a colonial spider, Metabus gravidus. Anim. Behav., 23, 560-567.
Chamberlin, R. V. & Gertsch, W. J. 1958. The spider family Dictynidae in America north of Mexico. Bull. Am. Mus. Nat. His., 116, 1-152.
Chrysanthus, F. 1935. Hearing and stridulation in spiders. Tijdschr. Ent., 96, 57-83.
Diguet, L. 1915. Nouvelles observations sur le mosquéro ou nid d’araignées sociales. Bull. Soc. Nationale Acclimatation, France, 16, 240-249.
Frings, H. & Frings, M. 1966. Reactions of orb-weaving spiders (Argiopidae) to airborne sounds. Ecology, 47, 578-588.
Gertsch, W. J. 1949. American Spiders. New York: Van Nostrand.
Jacson, C. C. & Joseph, K. J. 1973. Life-history, bionomics and behaviour of the social spider Stego-dyphus sarisinorum, Karsch. Insectes Sociaux, 20, 189-204.
Kaston, B. J. 1965. Some little known aspects of spider behavior. Am. Midi. Nat., 73, 336-356.
Krafft, B. 1970. Contribution à la biologie et l’éthologie d’Agelena consociata, D. (Araignée sociale du Gabon) n. Biologica Gabonica, 6, 308-367.
Krafft, B. 1974. La tolérance réciproque chez l’araignée Agelena consociata D. Proc. 6th Int. Arach• Cong., 107-112.
Kullmann, E. 1968. Soziale Phaenomene bei Spinnen. Insectes Sociaux, 15, 289-298.
Kullmann, E. 1972. Evolution of social behavior in spiders. Am. Zool., 12, 419-426.
Kullmann, E. & Zimmerman, W. 1971. Versuche zur Toleranz bei der permanent-sozialen Spinnenart Stegodyphus sarasinorum, Karsch. Proc. Arach• Cong. Inter., 5, 175-181.
Liesenfeld, F. J. 1961. Über Leistung und Sitz des Erschütterungssinnes von Netzspinnen. BioL Zbl., 80, 465-475.
Maynard Smith, J. 1976. Evolution and the theory of games. Am. Sei., 64, 41-45.
Millot, J. 1949. Araneae. In: Triâté de Zoologie (Ed. by P. P. Grassé), pp. 589-742. Paris: Masson et Cie.
Nielsen, E. 1932. The Biology of Spiders. Copenhagen: Levinand Munksgaard.
164
ANIMAL BEHAVIOUR, 27, 1
Parry, D. A. 1965. The signal generated by an insect in a spider’s web. J. exp. Biol., 43, 185-192.
Rovner, J. S., Higashi, G. S. & Foelix, R. F. 1973. Maternal behavior in wolf spiders: the role of abdominal hairs. Science, N.Y., 182, 1153-1155.
Shear, W. A. 1970. The evolution of social phenomena in spiders. Bull. Brit. Arach. Soc., 1, 65-76.
Szlep, R. 1964. Change in the response of spiders to repeated web vibrations. Behaviour, 23, 203-239.
Tretzel, E. 1961. Biologie, Oekologie und Brutpflege von Coelotes ter restris. Wider. Z. Morph. Oekol. Tiere, î., 49, 658-745. IT. 50, 375-542.
Walcott, C. 1963. The effect of the web on vibration sensitivity in the spider Acharanea tepidariorum, Koch. J. exp. Zool., 40, 595-611.
Walcott, C. 1969. A spider’s vibration receptor: its anatomy and physiology. Am. Zool., 9, 133-144.
Walcott, C. & van der Kloot, W. 1959. The physiology of the spider vibration receptor. J. exp. Zool., 141, 191-244.
Witt, P. 1975. The web as a means of communication. Biosci. Comm., 1, 7-23.
(Received 21 March 1977; revised 9 August 1977;
MS. number: A2029)