Images Collection
Read OCR Digitized Article Text
NOTE: This plain text article interpretation has been digitally created by OCR software to estimate the article text, to help both users and search engines find relevant article content. To read the actual article text, view or download the PDF above.
Anim. Behav1977,25,694-712
WEB-SITE SELECTION BY ORB-WEB SPIDERS, PARTICULARLY ARGIOPE AURANTIA LUCAS
By FRANK ENDERS*
North Carolina. Mental Health Research Division, Raleigh, N.C. 27611
Abstract. The entire behaviour of selection of web-site by Argiope aurantia is briefly described. Data on the following characteristics of the location of webs are provided: height in the vegetation, orientation of lean of web, use of different types of vegetation within a habitat, and abundance of insects at the site. In the laboratory, araneid spiders tested prefer higher light intensity and low humidity. The choice of height by immature A. aurantia is experimentally related to the height at which the spider begins its search for a web-site (point of release in cage), and to the covering of the cage (reduction of wind). Differences of these animals with Argiope trifasciata Forskal and with adult A. aurantia released in cages are reported and related to differential use of microhabitats. The behaviour of selection of website is discussed with regard to availability of prey and to predation pressure, comparisons being made to other groups of animals.
The habitat of an animal is the place where it lives (Odum 1959). Much has been written about selection of habitat by vertebrate animals (Klopfer 1962; Wecker 1963; Sale 1968, 1969a, 1969b). Less is known about this behaviour as observed in invertebrates (Meadows & Campbell 1972) and especially in spiders (Duffey 1966). Particular species of web spiders typically place their webs in certain situations (McCook 1889; Savory 1930; Davidson 1932; Tilquin 1942; Lowrie 1948; Barnes 1953; Luczak 1963 ;| Berry 1967; Turnbull 1973; Enders 1973; Riechert 1974). There is only one detailed study of the precise location of webs, within a habitat (Riechert 1974). No general, integrated behavioural explanation has been made for both the choice of habitat (coarse-tuning) and choice of web-site (fine-tuning). Savory (1930) and Tilquin (1942) stated that physical factors determine very precisely where a spider locates its web, but these authors did not describe exactly how such mechanisms could operate under field conditions ; in fact, to this date, there is no published description of what web spiders actually do when off a web and presumably seeking a new web-site. In contrast to the opinions of Savory (1930) and Tilquin (1942), McCook (1889), from careful field observations, already suggested that the exact spot where a spider actually builds its web depends, proximately, upon chaflce events, but, ultimately, upon an internal preference, which presumably has evolved in response to the available resources in the environment.
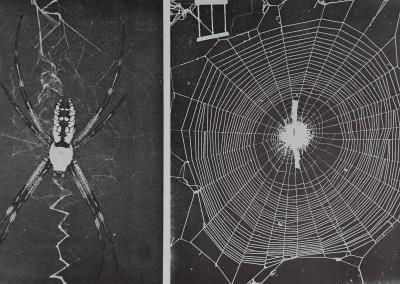
Here, I present original observations on the web-site used by one orb-web spider species, Argiope aurantia Lucas (Araneidae; Plate II, Fig. 1), within a plant community. I describe what this spider does during its choice among plant communities and what it does to choose a particular web-site within a plant community; in earlier papers I described the natural history of this spider (1973, 1974) and presented some information on the factors that influence change of web-site. Finally I here discuss the general differences in the way different species of web spiders, different kinds of spiders (web or nonweb) and different animals actually choose a place to live.
Web-site Used by Argiope aurantia and by A, trifasciata
Methods
A random sample of web sites actually used was obtained by visual search of plots along transects up the slope of road cuts along an interstate highway in Raleigh, North Carolina. The principal cover of the area was Sericea lespedeza, a perennial plant which dies back to the ground each year. Spiders of the genus Argiope had previously been found to be abundant here, so that a random sampling (stratified along the total length of road cut, with a random start) was practical. The study area and methods are described in more detail elsewhere (Enders 1972, 1974).
For data presented below, the coverage by the main different plant types was measured for each plot searched for spiders, and the direction of lean of almost every web was recorded. Most
♦Present address: 201 D Taylor Street, Raleigh, N.C. 27607, U.S.A.
694
Enders, Anim, Behav.% 25, 3
PLAT® II
Fig. 1. The spider Argiope aurantia and its web. The three vertical lines of the marker in the web photo span 4 cm.
ENDERS: CHOICE OF WEB-SITE

ENDERS: CHOICE OF WEB-SITE
695
araneid webs are attached to lean slightly from the vertical, the spider preferentially hanging from the ‘under’ surface, at the hub (Plate II, Fig. 1). In samples taken in 1970, the insects at each plot were sampled by five sweeps with a net (30-cm diameter); the numbers of arthropods (including insects and spiders) thus taken from areas immediately to the side of each plot searched for Argiope spiders gives an estimate of the abundance of food for Argiope spiders at a web-site. Data on heights (distance from hub to the ground) at which webs were placed in samples from 1970 (partially analysed in Enders 1974) are here re-analysed to show differences among instars of each species. Correlations of web presence with vegetation type and insect abundance were calculated. The relative use of plants as attachment points for the silk of the webs is also calculated from data on the actual attachment points of the webs; relative use is calculated as the number of webs found attached to a particular kind of plant divided by the total area covered by that plant in the plots with webs.
Results
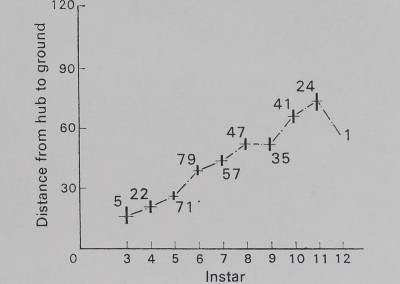
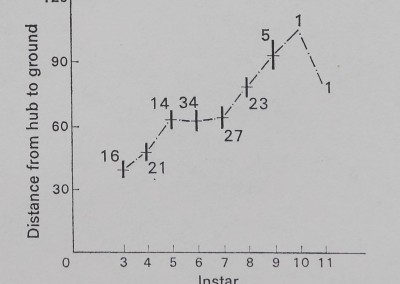
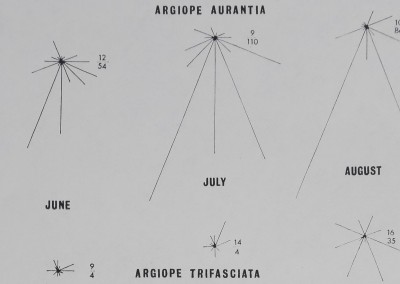
The height at which the web is located increases with instar for A. aurantia (Fig. 2). A similar increase in height occurs with instar for A. trifasdata (Fig. 3). The direction of lean of the webs of A. aurantia was generally such that the web leaned downhill (Fig. 4). Generally, the web of this species was also leaned away from dense vegetation. No such orientation of the web of A. trifasdata could be seen (Fig. 4). [Chi square (not corrected for continuity; Snedecor & Cochran 1967) indicates (P < 0*005) that the orientation of A. aurantia is statistically significant in every month, more downill than uphill; for A. trifasdata there was no statistical difference in May, June and September, and the differences in July and August were opposite in direction, with higher probabilities (0*05 to 0*025, and 0*01 to 0*005) of being due to chance.]
The number of each species of Argiope was positively correlated with the total number of arthropods taken near each plot in 1970 (Table I). The number of Argiope spiders on a particular plot was not correlated with the number of spiders (mainly Salticidae and Thomisidae) taken by sweeping (Table I). Some statistically significant positive correlations occur between the number of Argiope webs on a plot and the percentage cover of the plot by living lespedeza (P between 0*05 and 0*01, Table I). No significant correlations were observed between the distance
(or percentage distance) up a slope (transect) of a plot and the number of Argiope spiders found on the plot.
Argiope aurantia spiders show a general preference for dead lespedeza for actual attachment of webs. This species also prefers trees, shrubs and weeds in the rather uniform lespedeza stands, especially at the end of the year (Table II). In contrast to these preferences, for grasses and living lespedeza a relative use of even 1*5 was not reached. Data given in the table are not sufficient to be convincing on this point due to the small number of adults which are available in the random sample; also, a statistical test is
Fig. 2. The height at which webs of A. aurantia were located in the field. The vertical bar at each data point represents 1 se on either side of the mean, which is represented by the horizontal dash. The numbers on the graph represent sample size.
Fig. 3. The height at which webs of A. trifasdata were located in the field. The vertical bars represent 1 se on either side of the mean, which is represented by the horizonal dash. The numbers on the graph represent sample size.


696
ANIMAL BEHAVIOUR, 25, 3

Fig. 4. The directions toward which the webs of A. aurantia and of A. trifasciata leaned. The top of the figure is uphill. The numbers to the right of each diagram indicate the total number of webs leaning uphill as the numerator, and that downhill, denominator. The length of lines radiating from the centre of each starburst pattern represent the number of animals recorded to have webs leaning in that direction.
Table I. Correlations Between the Numbers of Argiope aurantia and of A. trifasciata Counted on Plots and Characteristics of those Plots. Perslope is the Distance along a Transect of 1-m2 Plots, Relative to the Total Distance from Edge of Road to the Top of a Lespedeza-covered Road Cut. Perlesp is the Per Cent Cover of a Plot by Living Lespedeza Plant. Argtri is the Number of A. trifasciata Counted on a Plot, Spiders is the Number of Spiders Taken by Sweep Net Adjacent to the Particular Plot, and Total Prey is the Number of Insects so Taken
| Perslope | Perlesp | Argtri | Spiders | Total prey | |
| Argiope aurantia | |||||
| June | – 0-18 | H- 0 03 | fill 0-07 | H 0-14 | MË- 0*06 |
| July | + 0-01 | BR- 0-28** | + 0-17 | — 0*03 | + 0-03 |
| August | – 0-15 | 4- 0-26** | Bill 0-52** | n 0*08 | 4- 0-21* |
| September | 0 05 | -0-07 | -f 0-27* | – 0*04 | + 0-27* |
| Argiope trifasciata | |||||
| June | + 0-19 | H 0 04 | *r# o-ii | 4- 0-29* | |
| July | Mt 0-09 | 1 0*11 | 0*15 | – 0-02 | |
| August | mm- o oi | + 0-23* | + 0*17 | + 0*02 | |
| September | + 0-13 | 4- 0-20 | + 0-09 | Eg 0-12 | |
♦Correlation statistically significant at the 0*05 level. ♦♦Correlation statistically significant at the 0*01 level.
not entirely valid, due to accidental omission of data in the field (due largely to lack of time to obtain precise data on some plots and some spiders). But, additional proof is furnished by haphazard field observations: most adult A. aurantia were concentrated at breaks in the blanket of live lespedeza; such breaks were provided by patches of bare ground, ragweed, evening primrose and by woody plants. A point which I emphasize is that A. aurantia did not always move the few metres necessary to reach
those breaks in the mass of dense living lespedeza ; instead, many adults built webs that were virtually horizontal on top of solid lespedeza, which orientation contrasts with the preferred vertical orientation of the web.
Preferences in Gradients of Humidity Methods
Saturated solutions of salts (Winston & Bates 1960) in a chamber produced a gradient of humidity. Six dishes below a false floor of nylon
ENDERS: CHOICE OF WEB-SITE
697
Table n. The Relative Use of Plants as Attachment Points for Webs of Argiope aurantia in Lespedeza-covered Road Cuts. The Relative Use is Equal to the Number of Webs Found Attached to a Particular Kind of Plant Divided by the Total Area Covered by that Plant in the Plots with Webs. Area is the Number of Square Metres covered; Portions of Plots were Bare
| May | June | July | August | September | ||||
| Kind of Plant | Rel.use ( %) Area | Rel. use ( %) Area | Rel.use(%) | Area | Rel. use (%) | Area | Rel. use (%) | Area |
| Living lespedeza | 106 49-3 | 91 35*9 | 81 | 85*0 | 81 | 53*9 | 42 | 25*9 |
| Dead lespedeza | 162 29*2 | 143 17*9 | 201 | 15*2 | 653 | 0*9 | 181 | 3*9 |
| Grasses | 36 19*7 | 108 16*3 | 134 | 7*1 | 105 | 3*3 | zero | 2*4 |
| Trees and shrubs | 124 17*0 | — — | 449 | 0-4 | 67 | 1*5 | — | — |
| Weeds | 84 9*9 | 149 3*4 | 95 | 6*0 | 231 | 6*4 | 297 | 7.8 |
| Total no. of webs 136 81 (— total area covered by plants and bare areas) | 116 | 69 | 41 | |||||
mesh contained solutions of dry P2O5, NaOH, CaCl2, saturated Na2Cr2Û7, NaCl or water. These dishes should produce relative humidities in the series 0, 5, 25, 50, 15 and 100%. Exact relative humidities present above the saturated solutions at particular temperatures are calculable from tables in Winston & Bates (1960). At times a short gradient from P2O 5 to NaOH to CaCl2 was used, or one with only water and CaCl2; results of such tests with different gradient steepness (Amos 1969) were consonant with those reported below. Details of experimental technique are described in Enders (1972).
The actual chambers used were of varnished, waterproofed Masonite with a glass cover sealed with a foam rubber lining. The boxes were maintained at a constant temperature and the humidity at various points in the gradient was checked by the deliquescence of crystals of salts exposed above the false bottom.
After being given water to drink, a single spider was placed above the false floor of each gradient in the morning. In the afternoon, the spider’s position in its gradient relative to the dishes was recorded and the gradient was reversed by reversing the order of the dishes containing salts. During this procedure, if a spider did (rarely) change its position, the spider was returned to its original position. The salts were replenished as necessary. On the following morning, each spider’s position was recorded once more. The average humidity chosen by all spiders was then calculated on the basis of the humidity judged from the final position of each spider and the theoretical relative humidity calculated from Winston & Bates (1960). Data for any spider which had not moved from its
position the previous afternoon were eliminated from consideration.
Results
Fewer than 10% of animals did not move, these mainly at the lowest temperatures of testing. Animals which moulted in the chambers were also excluded from the data presented here ; but they showed the same humidity preferences, up to the time of actual moult, when the spiders were physically unable to locomote.
As summarized in Table III, the araneids, if not deliberately desiccated beforehand, all preferred the dry end of the gradient. Positions chosen were not only the ends of the gradient. Smaller spiders built entire webs in the chamber. Data additional to that in Table III indicate a general preference for the dry end of the gradient by araneid spiders: single Micrathena sagitata, Mangora maculata and Larinia (directal), two Eustala {anas ter a ?), three
Mecynogea lemniscata and fifteen second instar Araneus cornutus preferred the dry end.
An interesting verification of the efficacy of the gradient is that several Argiope aurantia, Araneus diadematus, A. cornutus and Eustala {anastera ?) left for several days in the humidity gradient changed their preference from dry to the wet; these spiders then preferred the wet end until dead. This unusual humidity preference could be changed back to the original preference by providing a drop of water to drink. This test also shows, incidentally, that spiders of this family cannot absorb sufficient water from air, even at 100% humidity, at ordinary air tempera tures (24 and 32 C).
698
ANIMAL BEHAVIOUR, 25, 3
Table HI. Humidity Preference of Several Araneid Spiders. X — Mean Choice of Relative Humidity.
N » Sample Size
| Temperature(Q | Argiope trifasciata | A. | aurantia | Araneus diadematus | ||||
| X | Range | N | X | Range | N | X | Range N | |
| 35-0 | 3-7 | 0-24-3 | 12 | |||||
| 32*2 | 6-4 | 0-25-7 | 9 | 7-3 | 0-25-7 | 23 | 4-6 | 0-25-7 12 |
| 23-9 | 3-5 | 0-30-1 | 22 | 1-4 | 0-57 5 | |||
| 7-2 | 14-8 | 0-39-0 3 | ||||||
Several non-araneids that lived near the ground in the same habitat as A. aurantia were tested: one adult female Lycosa punctulata (Lycosidae) with offspring and three Agelenopsis sp. (Agelenidae). All chose the wet end of the gradient at all times. Altough spiders could not reach water under the false floor, the young L. punctulata were more dispersed, and even left their mother’s back, in the wet end of the gradient; but they clustered tightly in the dry end, with one another, when the mother was removed. In general, while an attempt was made to test spiders of different sizes, especially in Argiope aurantia, A. trifasciata and Araneus diadematus, no difference in humidity preference between adults and very small immatures could be discovered. Also, whether a light was provided at night or not, no difference in humidity preference of araneid spiders occurred.
Preference for Light
Methods
Spiders were tested to determine their preference for light in the same way in the same boxes as described above, for humidity preference. The only difference was that half the gradient box was covered with opaque plastic, and, of course, the salts and water were omitted. Results
All araneids tested preferred the lit end. This preference was found to occur also when these half-covered boxes were exposed outdoors with no moon. Ten individuals of each of the following species (plus a score of several species of unidentified immature woodland araneine spiders) preferred light: Argiope aurantia, A. trifasciata, Araneus diadematus, and Mecynogea lemniscata. Different instars of A. aurantia, from the second to the adult, all preferred the lit end of the gradient. Painting over the eyes, and céphalothorax, even several times, and
poking out the eyes using an insect pin, all could not change the preference of three adult A. aurantia for light.
Influence of the Direction of Light on the Orientation of Lean of the Webs
Methods
Glass-covered boxes used for humidity tests were placed on their sides so that light entered one side from the glassed-in ‘top’. A desk lamp was left on at a distance, or a street lamp provided light during the night. The light was dim enough so that the spider could not be seen, and no heat buildup was detectable to touch on the outside of the glass of the chamber.
Results
Nine A. aurantia of second through fourth instars built webs. Eight leaned toward the light, the spider’s body on the side of the web toward the glass. As predicted from field observations (Fig. 4), a one-tailed chi-square test shows the orientation of lean of the webs is significantly related to direction of night-time light. In addition, three young A. florida (from Bladen County, N.C.) were tested and built webs in the direction predicted (exact probability only 0-0625).
I would include the results for A. florida with those for A. aurantia because they appear to be geographic replacement species (Enders 1974). For all individuals of both species, the exact random probability is 0-0032, or less, that one of twelve would lean their webs in a particular direction.
Choice of Height: the Response of Second Instar Spiders to Wind and Light
Methods
In May of 1971, second instar A. aurantia were removed from stored cocoons. When these
ENDERS: CHOICE OF WEB-SITE
699
spiders began to build individual webs, they were placed on soybean plants at the Central Crops Research Station at Clayton, N.C. At this time, the soybean plants were about 30 cm tall. During the afternoon of 23 June, spiders were placed on the lower third of the plants and after initial activity most spiders were found to rest on the middle third of the plants. No A. aurantia had been found in such fields, and any naturally occurring spiders of this species would have been at least an instar larger.
Experimental treatments included (1) placing a black opaque piece of plastic to cover one linear metre of soybean row, and (2) placement of translucent plastic the same way. The two sides of the covering were secured by pieces of wood after release of about 60 spiders in the centre.
Results
Measurements of locations of webs and web height (distance to hub from ground), and height of the vegetation immediately above the web were made the next day at 14.00 hours (E.D.T.). Light measurements averaged 2450 lux in the open, 1000 lux under translucent plastic, and 33 lux 10 cm from the ends of the opaque plastic with a maximum of 1 lux in the centre of the opaque plastic. From 9 to 20 % of spiders remained in a given plot, including animals that had not yet built orb webs the next day. A higher percentage remained in plastic-covered plots. Five webs were found under the opaque plastic, six under translucent, and 19 outside the limits of the plastic. Since possible web heights are truncated at about 3 cm (half the length of the face of the web), the web heights are not expected to be normally distributed. The Mann-Whitney CT-test (Snedecor & Cochran 1967) revealed that the control group of 19 animals did not differ in web height from a group of 37 naturally occurring second instars in lespedeza in May, nor from other second instars subsequently released in soybean and in natural old-field vegetation. There was also no significant difference between the web heights for the two groups of webs built under plastic sheets, opaque versus translucent. But there was a highly significant difference (P < 0*01) between the eleven webs built under plastic covering and the 19 control animals.
Similar results were obtained during release of hundreds of second instar A. aurantia in July in the same field (an experiment with a •different purpose, to be described by Enders &
Bradley, in preparation). And similar results were obtained for releases of spiders of instars as late as the fifth in natural old-field vegetation.
Therefore, it appears that generally reduced levels of light from opaque plastic covers did not change the height at which A. aurantia built, compared to the effect of translucent plastic. A few A. trifasciata and Mangora gibborosa built webs under plastic coverings by accident; spiders of these two species normally build webs high in herbaceous vegetation, and their web height was not affected by wind or light reduction.
Choice of Height: the Effect of Wind Reduction and the Effect of Height at which Spider was Released
Methods
Spiders were collected before the experiment began and were left at least overnight in the cages described below. Some data came from animals maintained in cages as long as a month. All animals received at least one house fly per week as prey, and water more often.
Cages were open-bottomed cylinders of screen wire 91 cm in height and 30 cm in diameter. These cages were sunk 5 cm into the ground and the bottom edge sealed by packing wet sand around the bottom. The cages were supported by stakes and string outside.
The two treatments of the factorial design experiment were:
(1) Point of release: release of spider either at top or at bottom of the cage. The spider was introduced in a manner so that it was left stationary within 10 cm of top or bottom after the cage had been set into the ground.
(2) Bagging: covering the cages with plastic to eliminate (or at least very greatly reduce) movement of air during the night.
The web was destroyed in the evening and one of four treatment combinations was applied between 19.00 and 21.00 hours: bagged and bottom released, uncovered and bottom released, bagged and top released, uncovered and released at top. On alternate days spiders were left undisturbed. Data were obtained on 12 different days in July and August of 1971. The plastic cover, if any, was removed before 11.00 hours the morning after a treatment; this avoided heat buildup. The distance between ground inside the cage and hub of the web was measured to the nearest 0-5 cm and is termed ‘web height’ here. Each spider was used as a replicate of the
700
ANIMAL BEHAVIOUR, 25, 3
experiment, receiving all four treatment combinations on different days.
The cages were placed in a garden in Raleigh, N.C., with open areas extending from 10 to 100 m in various directions, in a meadow above a stream. Tall trees were located nearby, but there was no heavy undergrowth, and the area within 10 m of the cages was kept mowed.
Spiders that escaped from cages provided only incomplete replicates, but showed the same pattern as data used in the analysis. Spiders usually did not build more than a rudimentary web the night of moulting, and data for days when spiders had moulted were excluded from analysis.
Spiders used were immature A. aurantia, from fifth to ninth instar. The first of three runs included two sets of data for the five animals that were tested twice. Adult A. aurantia and various ages of other araneid spiders also were tested under one treatment combination (unbagged, bottom release).
Since web heights are truncated at zero plus half the web diameter, and at 91 cm less half the web diameter, it is not surprising that the first two runs of this experiment showed significant heterogeneity of variance among treatment combinations (by Bartlett’s test; Snedecor & Cochran 1967), though the third run did not. The variances were homogenized by a logio transformation. However, the differences in level of statistical significance of the treatments between analyses using transformed and untransformed data were minor. Hence the results below are presented in the more intelligible untransformed form.
Results
For the immature A. aurantia the correlation coefficients between sets of data for animals that were repeated in the first run were very low, averaging 0*06 for all treatments, the greatest being — 0*43, not statistically significant.
Data from second instar spiders presented in the previous section predicted a significant effect of bagging, which was found here (Table IV). The significant effect of point of release is a useful method to block out extraneous variance. But, this latter effect also demonstrates the extent to which the place where a spider begins its search for a web-site can affect choice of website. Plainly this ‘chance’ or ‘historical’ effect on choice of web-site can be important in the field situation, as well as being statistically significant under experimental conditions.
A dozen adults of A. aurantia and a dozen A. trifasciata (both immatures and adults) were tested and built their webs touching the tops of the cages, under the unbagged, bottom release condition. The few adult male A. aurantia that built webs in the cages all built them high up; I remark this especially because they were several instars smaller and several millimetres shorter in length than immature females which were still building webs lower down, near the ground.
In one incomplete and two complete replications, two immature Gea heptagon (Argiopinae : Araneidae) showed the same pattern as immature A. aurantia: webs were highest in bagged and top-release of spiders, lowest in unbagged and bottom-released. These spiders also showed the same interaction of bagging with point of release: these spiders climbed downward to
Table IV. Effects of Bagging and Release Height on Web Height, in Immature A. aurantia. The First Run Includes 20 Replicates, the Second, 13, and the Third, 14. Both Treatment Means and Factorial
Effects are in centimetres
Treatment means (height above ground at which web is placed)
| 1st run | 2nd run | 3rd run | all data | ||
| Released at bottom | Not covered | 20*9 | 30*4 | 22*6 | 24*1 |
| at bottom | Bagged | 28*5 | 39*8 | 25*8 | 30*8 |
| Released at top of cage | Not covered | 34*5 | 38*7 | 22*5 | 32*1 |
| at top | Bagged | 57*0 | 48*1 | 42*7 | 50*3 |
Factorial effects (increase of web height as a consequence of)
Place released 13 *7 * *
Bagging (wind removal) 12*5**
Interaction 5 •7*
♦Significant at the 0*05 level. ♦Significant at the 0*01 level.
ENDERS: CHOICE OF WEB-SITE
701
build webs low in the unbagged condition, but would not climb as much to build in the bagged condition.
Time of Web-building Behaviour and Behaviour of Spiders off the Web
Methods
I spent several nights in the field to observe web-building by Argiope aurantia of different ages. During this time I made many deliberate observations of spiders on and off webs, using as little light as possible. Since completing the field-work for this study, I have spent more than 1000 night-time hours collecting and observing arachnids in the field, in habitats ranging from deserts to jungles, and was able to make many incidental observations of araneid spiders. Since there is no published description of what the araneid spider does when off the web and presumably seeking a web-site, I have taken it upon myself to present a summary of some representative observations, even though they are not quantified in any precise way.
Results
In the laboratory A. aurantia builds its webs during the night, but can often be seen completing the sticky spiral or radii in the morning just after the lights have been switched on by an automatic timer. In the field A. aurantia builds webs at night, completing the web in the morning, at times. However, the second and third instars often take two mornings to complete a web. Except for one occasion after a storm had destroyed many A. aurantia webs and a marked individual was seen rebuilding its web at the same site, and except for the building of‘radii’ by spiders taken from their webs, all web-building activity of this species occurred at night or a few hours after dawn : of ten second instar spiders building at the same site, none had completed webs at 5.20 hours, four had done so by 8.00 hours, ten by 12.00 hours, and one remained on radii till the next day. (Of course, some araneines (round-bodied araneids) regularly begin building webs shortly before nightfall.)
Results of observations can be summarized as follows, for both species of Argiope, and for some other araneids observed by me:
(1) Spiders which remain at the same site take up wedge-shaped sections of the web at various times during the night, beginning after dark. New radii are placed before the entire old web has been taken up. But, by the time when the entire old web is already taken down,
not all radii have been replaced. The old frame seems not to be replaced; replacement or partial replacement of the frame occurs, but such is more difficult to observe than are new radii.
(2) Released spiders may be considered equivalent to those few spiders observed that had voluntarily left an old web-site. Released spiders climb to the top of the vegetation, in stages if the vegetation is tall. There may be an initial period of immobility after release, even on the ground. ‘Questing’ (waving the front legs in the air like a tick; Camin 1963) occurred when the spiders reached the top of the vegetation. This behaviour is performed both by spiders that remained at the high point to build webs and by those that dispersed out of the area later during the night. Many spiders put out silk and hung upside down on it, even building what could be assumed to be the ‘radii’. This latter behaviour signalled that the spider would be found in the same place the following morning, on a web. But no other behaviour was noted which might serve the spider as a prefatory method of measuring the environment. Were the vegetation too flexible, the spider’s motion bent it under. Some spiders walked off on silk they put out, instead of becoming still. Moving spiders, even adults, often seemed to be initiating movements only when struck by a puff of wind, but many spiders subjected to the same wind movements did not begin to move.
Observations of spiders in experimental cages gave some data on the time at which the website was determined. Immature A. aurantia released in outdoor screen-wire cages build webs higher up in cages that are covered with plastic than in uncovered ones. Thirteen immature A. aurantia were therefore left covered until 4.00 hours and were then uncovered. That afternoon, the spiders that had at least a Y-structure (Witt, Reed & Peakall 1968) at 4*00 hours all had webs located within 5 cm of the location measured at 4.00 hours. This included eight A. aurantia with frame plus radii, two with at least a Y-structure but no more than three radii, and one Gea heptagon with a Y-structure. But, the three A. aurantia which definitely did not have any Y-structure (two on a single line of silk and one on the wire of the cage) had all changed position, to build webs much lower down than the location recorded at 4.00 hours, i.e. had shifted downward more than half the web diameter, at least 20 cm. The observations (summarized above) from unconfined spiders also show considerable natural
702
ANIMAL BEHAVIOUR, 25, 3
variation in the time at which the spider places the Y-structure, during web-building. These observations suggest that the location of the web is determined by the placement of a Y-structure. The Y-structure alone is difficult to observe under field conditions, but the presence of many radii can readily be seen, and this presence of many radii may have to serve as a field mark for the placement of the Y-structure. The Y-structure may be relatively ephemeral, since additional radii and much of the frame is put down shortly after the Y-structure is placed (Witt, Reed & Peakall 1968), this besides the difficulties of seeing the few silken threads without using sudden, strong lighting which may disturb the building spider.
Discussion Ecological Context of this Study
The form and building of the web of A. aurantia have been described (Reed, Witt & Scarboro 1969; Benforado & Kistler 1973). Predatory behaviour (Robinson & Olazarri 1971) and prey actually taken (Robinson & Robinson 1970) by the ecologically equivalent (Enders 1974) Latin American species A. argentata (Fabricius) are known; and there are recent studies of predatory (Harwood 1974) and escape behaviour (Tolbert 1975) of A. aurantia. Such information can help place the behaviour of selection of web-site in context; namely, what is the relationship of the orb-web spider to its environment ?
Any web spider is a polyphagous trapping insectivore (Bilsing 1920). The web of the spider also increases the amount of information in the ecosystem via the ethological or cultural channel (Margalef 1968, p. 98), which is distinct from the ecological (environmental) and genetic channels of information. Due to the use of the web in addition to poison, web spiders are effectively larger predators, relative to the available prey and their own physical size, compared both to equally-sized chewing predators such as mantids (Holling 1964) and to poison-using predators such as non-web spiders. Elsewhere, I discussed the manner of hunting (‘strategy’) of orb-web spiders in comparison to other insectivores (1975b) and in relation to a semi-quantitative outline of the food types available to insectivores (1977). Unfortunately, as foraging theory (Kennedy 1950; Schoener 1971) is not as well-developed mathematically, as quantum theory in physics, we cannot generate precise predictions of the behaviour of spiders from these arguments.
Moreover, just as it seems to be cheaper to do some experimental work in chemistry rather than using pure quantum theory (Dewar 1975) to predict the behaviour of chemicals, so too, for many years to come, we will remain unable to predict the behaviour of organisms from a knowledge of their basic ecology, but must be content with observation without prediction. In the case under consideration, web-site selection, both the ‘fundamental’ ecology of the spiders and the steps of logic leading from ecology to predictions of behaviour are so insecurely known that no precise predictions were possible at the start of my investigations reported here.
Nevertheless, physiological constraints on animal species are generally less important than availability of resources to determine selectivity for habitat, particularly in the case of this study, done in the centre of the broad latitudinal geographical range of A. aurantia. The upper temperature limit for this species and for A. trifasciata was experimentally determined to be near 45 C (unpublished data), quite high. Hence, climate was anticipated to have little influence and little control of the choice of website in this species.
Other data indicate that A. aurantia may have less stringent requirements for habitat than other, more-specialized spiders. As argued in Enders (1975b) the very size of the individuals of this species may force it to be less specific for habitat. This spider does occur in a range of early successional habitats (Enders 1973). And, for its size, the spider A. aurantia produces exceptionally many eggs (Enders, 1976b), These data suggest that my results on the behaviour of A. aurantia must be interpreted as coming from a species of ‘r’ character (Pianka 1970); the outbreaks of populations of this species reported by Levi (1968) also support this interpretation. But, the idea of r-adapted and K-adapted species generates no specific predictions; I may have only added to the confusion by suggesting that r-adapted species are time-budget species and K-adapted species are energy-budget species (1975b). There is a ferment in the area of behavioural ecology, but the theory, while developing in many ways at once, is still disorganized and cannot successfully predict any but the most rudimentary generalizations in situations, as with spiders, in which we lack the observational data on related species which make the ‘predictions’ of theoreticians reliable, but also foregone conclusions.
ENDERS: CHOICE OF WEB-SITE
703
in this situation we are reduced to very elementary generalizations. Since most spiders are neither directly limited to plants nor to specific prey for their sustenance, I thought that, like other predators (birds, etc.), spiders may select habitats on the basis of physical form of the environment (Elton 1966; Colebourne 1974) rather than being limited to plant species per se, and this less-specific use of habitat may be a general phenomenon among spiders (Duffey 1966).
Web-sites Preferred by A. aurantia within a Habitat
Grass was less-used; unless several stems were tied together, as sometimes occurred, grasses lack sufficient strength to support the larger Argiope individuals. Living lespedeza similarly was not preferred for web attachment (Table II). Living weeds, as well as living lespedeza in the field, seemed to have more insects, while grasses and woody plants had lesser abundance of insects, and dead plants, the least. Since insects were seen to be more common in living dense lespedeza than in other vegetation available on the study area, the positive correlations between percentage of cover of plots by living lespedeza and the numbers of Argiope spider webs found in those plots (Table I) may indicate a positive influence of prey abundance on growth and survival of Argiope spiders. The positive correlations between prey abundance and numbers of Argiope spiders (Table II) can also be interpreted in this way. Clovis Moore (unpublished) has data which similarly show a correlation between prey capture and presence of an orb-web spider (Nephila clavipes), and he supposes the correlation may arise via some behaviour of the spider leaving unproductive web-sites. Enders (1976c) found no direct positive behavioural response of web-site tenacity to prey-catching in A. aurantia, in several experiments in both field and laboratory. Instead, the positive effect of insect abundance on the numbers of A. aurantia could easily be mediated by differential predation on smaller and less well-fed Argiope spiders. Such predation by Mimetus (Mimetidae) spiders, which occurred on the lespedeza study area, has been observed, especially when undersized A. aurantia were released (Enders & Bradley in preparation). In addition to mimetid spiders, which attack web spiders almost exclusively, jumping spiders (Salticidae) may be involved (Enders 1974,
1975b; Tolbert 1975) in predation upon undernourished A. aurantia.
In addition to the correlation coefficients presented in Table I, I have calculated other correlations between environmental characteristics and numbers of Argiope spiders at a particular square-metre plot within my lespedeza study area, only some of which are presented in my thesis (1972). I also computed multiple regression models which explained more than 50% of the variance of numbers of A. aurantia among plots within the study area, on the basis of items originally chosen for measurement (plant cover, other Argiope species, potential prey, distance up slope). These statistics provided more instances of statistical significance than the one of twenty expected from a random assortment offldata and tests. But, no clear biological or behavioural conclusions could be drawn from most of the more complex multiple regression models, such as the highly significant correlation between large (2+ cm) potential prey and numbers of A. trifasciata in July but not in June nor August, arid so I have spared the reader those details. Instead, I have presented the patterns of behaviour that explain the correlations which make biological sense, in a unified explanation of web-site selection by the one species for which I have the most data.
Choice of Web-site by Individual Argiope aurantia
I have considered the relation of the actual web-site to factors suggested by previous workers (humidity, temperature, chance, food, vegetation structure, predators), and I did field experiments manipulating factors which might have a behavioural influence. My observations on the behaviour of A. aurantia generally agree with those of McCook (1889) in both detail and interpretation of the data.
This spider begins life as a second instar that emerges from the egg sac, and it gradually disperses from the egg sac, probably within 2 days of emerging. I enclosed both cocoon and emerging A. aurantia in nylon netting in the field, and observed marked fluctuations in the numbers of spiderlings outside the cocoon but inside the netting. This confirms Wilder’s (1873) statement that this species can return to the cocoon. Contrary to Wilder (1873), but in accord with McCook (1889), I found no evidence for cannibalism at this stage (no marked differences in size among emerging animals). (A. trifasciata differed from A. aurantia
704
ANIMAL BEHAVIOUR, 25, 3
by remaining grouped above the cocoon for several days after emerging.)
The second instar spiders emerging from the cocoon climb upward to the light. Tilquin (1942) demonstrated the positive response to light seems stronger than the negative response to gravity, in other species of araneids. By placing light to the side of emerging A. aurantia spiderlings, I found that, once the spiders have laid down silk above a cocoon, light does not attract as many young to the side (which may be a laboratory artifact, since not so much silk is produced in the field, before the spiders balloon away). From the top part of the plant or other support, these spiders balloon away on silk (McCook 1889; Kaston 1948). I found no difference in the fraction of a group which dispersed in this aerial fashion after confinement of different groups in relative humidity from 5% to 100% (for several days) immediately prior to their normal dispersal. At this age the spiders very readily play out silk when one blows air at them. Natural gusts of wind on a warm spring day seem to be sufficient stimulus for A. aurantia spiderlings to disperse by ballooning, i.e. if they are of the proper age or physiological state. Explanations of ballooning cannot be based only on external conditions such as change of humidity (Duffey 1962).
Eventually, the spider builds a web where it comes to the earth’s surface, whether it has ballooned once, several times, or not at all. In dense vegetation, about 13% of the spiderlings remain at least several days within a few metres of the point of departure (Enders 1973). To judge from the high, uniform densities of webs of second instar A. aurantia noted in lespedeza, more must remain at slightly greater distances. There may be morphs which disperse and those which do not, such as found in moths by Wellington (1965) and others. Specifically, Benforado & Kistler (1973) found both slow-growing and fast-growing individuals of this species of spider, but the rate of dispersal by slow- and fast-growing individuals of spider has not yet been investigated.
The first web of A. aurantia thus comes usually to be placed in an area sheltered from wind, within 15 cm of the ground (Enders 1973, 1974; Fig. 2 and p. 698) Data presented above indicate, that the spider will build webs higher up if there is less wind, both in cages and in the field.
1 also induced some second instars to build webs
2 m off the ground in the crotch of a mulberry tree and second instars released in August built
fully 30 cm off the ground in that year’s dense, tall lepedeza. Finally, once the spider builds a web in such ‘unnatural’ areas (as in woodlands; Enders 1973), it may be found there for several weeks, and spiders even achieve growth in the ‘inappropriate’ vertical or horizontal habitat. These experimental results indicate that many spiders collected in ecological surveys may be collected in situations only due to the (chance) nearby presence of a focus of dispersal of the species.
Second instar spiders are twice as prone to change web-site as adults (Enders 1975a). These early instars also differ from later instars in taking two nights to complete the web (reported above). Limited silk production by very young spiders might prevent completion of the web. The initial phase of non-building or prolonged building of webs was probably shorter in nature (though observed there), and this period might normally represent the time spent to disperse by ballooning.
Responses in gradients indicate that spiders not on the web move toward light and away from humidity. Due to the failure of my attempts to interfere with the capacity of A. aurantia to move toward the light, I cannot prove that adults of this species leave shaded woodlands (Enders 1973) due to the lack of light in that habitat, though such is an appealing hypothesis. The responses to light and to humidity could serve not only for selection of habitat, but to bring the spider into a space open enough and large enough for a web. It is still not known whether the spider fits its web to the web-site, though possibly the spider can do so by making the web smaller, as necessary, retaining the surplus silk in its body. A. aurantia and Neoscona sp. (arabescal) araneid spiders did leave web sites which had been divided by twigs placed through the area of the hub.
The positive response to light and negative response to humidity, plus the tendency to climb upward (which occurs even in closed laboratory containers), together bring the immature A. aurantia to higher regions where there is wind. My observations of behaviour, the choice of web height by immature spiders of this species, and the desertion of web-sites after removal of nearby vegetation (Enders 1976c) all suggest that these immature spiders off the web must often jump off the vegetation in response to wind. For all practical purposes, for any small spider, this behaviour is indistinguishable from ballooning behaviour.
ENDERS: CHOICE OF WEB-SITE
705
Conceivably, the spider balloons until it has used all but some fixed amount of its energy reserves from the egg. A similar hypothesis obviously can be contructed for young immature spiders leaving a web-site, and energy reserves may be related to the rate of growth of individual spiders (Benforado & Kistler 1973).
Since the middle instars of A. aurantia complete their web in the early morning, there usually would be insufficient thermals or other air movement to support vertical (long-range) ballooning by spiders of this size. Once I saw a middle instar A. aurantia ballooning horizontally at mid-day. The presently available data indicate that the dominant proximate influence on the immature A. aurantia to determine its web-site is the amount of wind at the place where it alights; the spider will leave a plant community (Enders 1973,1976c) or move to a lower position to build its web, if the wind in the vegetation is too great.
Web destruction (by wind or by animals, both rare events) does cause the spider to leave the web-site (Enders 1976c). Web destruction would be relatively more important an influence on older spiders, which show less ‘intrinsic’ tendency to change web-site, even in the laboratory (Enders 1975a). This influence of web destruction might help explain the preference for dead vegetation found in Table II. Living vegetation bends a great deal in the wind, and this rips up webs, even if the vegetation is able to support the resting weight of the spider when it is building the web. On the other hand (Duffey 1962), dead materials do decay and can snap in the wind.
It is clear from my observations that the largest, adult A. aurantia do not balloon vertically. They apparently often do not even use drifting, wind-borne silk to set the first part of the frame, such as spiders with more-aerial webs (genera Eustala and Neoscona, Araneidae) regularly do. It is difficult to distinguish silking to balloon from silking to set down the frame of the web; the distinction may be purely academic. Differences among species in silking behaviour may prove significant for the differences in choice of habitat among araneids. The woodland species of spiders (plus a desert species) which generally refuse to build webs, or build them rarely in 30-cm diameter outdoor screen cages, are the very species which use long strands of silk to suspend the frame of the web in a large ‘space, e.g. Micrathene.
By the time A. aurantia mature, either as a small male of the sixth, or a large female of the twelfth instar, there is no longer any demonstrable negative response to wind. The spider then seems to build its web as high as it can climb in open areas. The lack of a well-developed tendency to silk when first putting out the frame, and the very weight of the (large) Argiope spider restricts this species to the top of the main mass of vegetation. In woody plants, however, both A. aurantia and A. trifasciata can build webs quite high up.
Physiological Control of Web-site Selection by Argiope aurantia
The most prominent features of web-site selection by A. aurantia are that the spider is at first restricted to sheltered sites, apparently by wind, and that later in life the spider climbs as high as possible. In A. trifasciata wind reduction is not so important for choice of web-site (data above, and Enders 1974, 1976c). Other factors, not yet obvious, may also be important. The spider will leave a web-site if a stem is placed through the centre of the web and attached in place; the spider leaves during the night, and not immediately. This suggests that the spider, at night, might respond to additional inputs received during the day and stored up. Nonetheless, the responses to wind, light and humidity, plus the tendency of the spiders to climb upward, can explain the selectivity for web-site observed in my field data.
Sale (1969) describes depth and cover selection by juvenile fishes (manini) as dependent upon an increased level of exploration in an unsatisfactory habitat. He states that an appetitive behaviour continues until an appropriate (inherited) consummatory stimulus-situation is encountered. Wecker (1963) and Klopfer (1962) have demonstrated that the preferred stimulus, at least in certain species, may be learned during early life.
The orb-web spider walks away especially when disturbed by the wind, and such ‘appetitive’ behaviour may continue until the spider reaches an appropriate stimulus condition, no wind. Appetitive behaviour also occurs when the spider climbs upward (or toward the light), until it reaches the top (as an adult); to judge from the spider’s behaviour, the top is defined by the spider’s inability to reach something higher with its leg or with silk. There is one main complication preventing a simple-minded application to spiders of Sale’s statements; the web
706
ANIMAL BEHAVIOUR, 25, 3
spider is in no position to explore once the web is fixed. My observations indicate that most appetitive exploratory behaviour occurs at night. In formal terms, the appetitive behaviour therefore may be temporally dissociated from the incoming stimulus that was dissonant with the appropriate internal standard for that stimulus; also, the dissonant stimulus (wind) may often reach low levels or be absent during the night when the spider actually makes a choice of a new web-site.
If the level of appetitive behaviour is controlled by available energy reserves (as in moths; Wellington 1965), then differences in previous feeding conditions might help explain why certain A. aurantia, when released, build webs immediately, while others show more appetitive behaviour. Memory, genetic factors, or pure chance might also help explain my observation of differences among individuals in the level of appetitive behaviour. Indirect measures such as the time at which the spider begins to take down its web, and whether it takes the entire web down or begins to replace portions right away, may provide a better measure of exploratory behaviour than any direct measure of movement.
Both McCook (1889) and Tilquin (1942) say that spiders show a great attraction for silk (‘sericophily’). Spiders will remain in the webs of other individuals or other species, if placed there, unless the spider is too heavy for the web (Peters 1970; Enders 1974). Attraction for silk was suggested to explain cases of web invasion (Enders 1974). The presence of silk in the silk glands or the presence or absence of silk at a web-site or both may be very important to control the changes of web-site from day to day, e.g. the absence of silk at the site, as a result of destruction of the web, might thus explain the lower web-site tenacity after web destruction (Enders 1976c), since the web-site otherwise is just as useable as before. Witt, Reed & Peakall (1968) describe that the araneid spider maintains the catching area of the web upon starvation, while bodyweight goes down; this suggests the interchangeability of silk protein and protein of the body. Therefore, if the webs in the field grow in size as they do in the laboratory, the spider could gradually find itself in a site too small for its silk supply, and could become increasingly ‘dissatisfied’ with the site because of the excess of silk. Some of the excess silk could be put into the barrier web, which seems not to be built the first night by A. aurantia.
But, direct approach of this hypothesis may be impossible because it requires one to measure the silk supply of a spider which is free to change web-site.
In general, my observations on the behaviour of the araneid spider are in agreement with those of Sale (1969) on fish. The spider off the web seems undirected, relative to particular web-sites, and depends upon chance events to determine the suitable web-site, as McCook (1889) has pointed out. Ultimately, however, the spider has some internal evaluation of the web-site: the use of light and of wind-reduction provides a high probability that chance encounters with particular web-sites will result in discovery of a satisfactory location for the web. All information so far available for A. aurantia, such as rearing it in laboratory conditions, indicates that the internal standard used to evaluate the web-site is inherited, only. The standard has been shaped by the external selective pressures of competitors (Enders 1974), physical factors, and resources such as predators or food that are important to the spider’s individual survival. It is not certainly known whether web-sites can be a limiting resource for web spiders. My present thinking is that food alone is the resource, which can predict the internal standard used by animals to select their habitats. Choice of website clearly can only occur if, on the average, the increased prey intake possible at a better web-site more than exceeds the increased predation rate on web spiders off the web (Enders 1976c). A general discussion follows, focussed upon the availability of the food resource and predation pressure as they affect the evolution of site selectivity.
Choice of Web-site by Other Spiders
Obviously there are trivial physical limits to spider distributions such as extreme heat or cold; such factors would eliminate the prey resource (Enders 1977) at the same time as the spider, so there would be no reason for the spider to evolve to fit such useless habitats. Another trivial limit is that the absence of physical structure suitable for attaching a vertical orb web directly limits araneid spiders from such areas as grasslands (pointed out by Muma & Muma 1949; Cherrett 1964; Schaefer 1970).
More significantly, Duffey (1966) pointed out that some species (including even some non-web spiders) are ‘diplostenoecious’, to occur in habitats with similar physiognomic characteristics, despite the presence of very different
ENDERS: CHOICE OF WEB-SITE
707
moisture and/or temperature regimes in the two places used, such as marshes with tussocks and beaches with boulders. It is reasonable to suppose that wind controls the distribution of these spiders among habitats just as it seems to control the distribution of A. aurantia (which also occurs both in dunes and in marshes; Lowrie 1948; Levi 1968). Due to araneid spiders’ tolerance of such differences in microclimate, as argued in Enders (1973), it is difficult to credit arguments offered by Almquist (1973) and by Riechert, Reeder & Allen (1973) that behavioural response to temperature and humidity (which are extremely variable over the course of a day) can determine the precise location of spiders. There is no careful study of habitat selection by a non-web spider. Direct and detailed behavioural or experimental field studies would almost certainly show that the family Agelenidae (observed by Riechert et al. 1973; Riechert 1974; Riechert & Tracy 1975) among the web spiders is close in its behavioural characteristics to non-web or burrowing spiders (as opposed to aerial web spiders); I state this because there is a diversity among the spiders not appreciated by most biologists, and because the Dyar’s constant, clutch size, and humidity preferences of funnel-web agelenids are, in fact, more like those of the non-web spiders (data in Enders 1976a, 1976b, and above,* respectively). The non-web and burrowing spiders are, at best, only marginally physiologically adapted for life away from the litter layer (Duffey 1962), and they capture prey less often than do web spiders (Enders 1975b). A great diversity of behaviour can certainly be anticipated among various families of spiders, in habitat selection, just as occurs in predatory behaviour (Enders 1977), but no such diversity has yet been demonstrated experimentally. Additional study is necessary before any definitive statements are possible.
Savory (1930) and Cherrett (1964) demonstrated preferences for the dark end of light gradients by members of pairs of species of araneids. The two spiders which preferred the dark live in houses and in caves. Meta merianae, the cave spider, also preferred moist places in humidity gradients (Cherrett 1964). The other two araneids tested by these authors, and all araneids tested by me, select light and dry parts of gradients. These other species live in woodlands, gardens and fields, the usual habitats for the great majority of individuals and species of Araneidae. Clearly the two species cited which
can locate their special habitats by the use of negative responses to light and positive responses to humidity are unusual cases for the Araneidae.
Selection of Web-site by the Convergently-evolved, Uloborid Orb-web Spiders
Eberhard (1971) concluded that an undirected response to excessive wind was most important in choice of web-site by Uloborus diversus (Uloboridae). But, his observations do not cover the range of stimuli such as summarized here, in my experiments on Argiope aurantia (Araneidae). I found the uloborid U. americanus typically in microhabitats similar to those of Gea heptagon and young A. aurantia, low in sheltered (and dark) places. And, unlike those argiopine araneids, the uloborid even occurred in barns. My observations in areas where Eberhard worked also suggest that Uloborus is not characteristic of open desert, but uses darker, more mesic areas provided by packrat nests. Therefore, I suggest that Uloborus species may show a positive response to humidity or a negative response to light, such as other web spider species which live in houses or caves; light also may serve Uloborus as a token for the lean of web, like for A. aurantia. I should point out that I have no idea what the adaptive significance of this characteristic of A. aurantia could be; even a direct experimental approach to the ecological significance of this orientation (as Barnes, Crisp & Powell 1951) might still leave us in the dark.
While Eberhard (1971) produced the first detailed study of the ecology of the web of a spider, there are many gaps in his published study, such as how the negative influence of prey capture which he describes can interact with the positive correlation between wind breaks and insect abundance which he suggests as a controlling influence on the distribution of his spider. Logically, the spider would then disperse away from the centre of prey abundance to which it had just reached by responses to wind and to light. Araneids tested in the laboratory by me moved away from the wind produced by a fan, rather than changing the frequency of webbuilding, which latter is the parameter measured by Eberhard.
Habitat Specificity and the Food Resource for Orb-web Spiders
The usual constellation of orb-web spiders in the habitat of a species of orb-weaver may explain the species-typical web-site, and so the
708
ANIMAL BEHAVIOUR, 25, 3
behaviour involved in reaching that web-site. This can occur both by the mechanism of interspecific interference competition (Enders 1974; realized niche) and by the evolution of the internal standard used by the spider to evaluate a web-site (fundamental niche). Riechert, et al. (1973) observed extra-web territoriality which can influence resource use in the case of any web spider which will leave the web ; territoriality also occurs in aggregated, coloriai orb web spiders (Lubin 1974; Buskirk 1975). Studies of territorial behaviour in solitary spiders, in relation to different levels of food availability are badly needed. Extra-web territoriality does not seem to occur in Argiope spiders, except for such cases, as evident from the data of Buskirk (1975), when the spider outside the web can vibrate the web support in such a way to transmit vibration to the web.
Ultimately, the species that live in the habitat of web spiders are controlled by the availability of food resources, which is affected by use of the resource by competing species. The presence of insectivores which also eat spiders (see next section) is probably correlated with the presence of insects which might serve the spiders as prey. In the early successional stages of plant! communities which are used by the Argiope species, primary and so presumably also secondary productivity is about the same as for forests (Golley 1972; discussed in Enders 1975b); due to vertical compression of the habitat by about an order of magnitude, I conclude that prey, per se (and predators), is probably present in excess for these spiders, in contrast to the lower availability of insects as prey that is possible for woodland web spiders (outside insect flyways). I therefore agree with Riechert et al. (1973) that competition for space by web spiders in such habitats with high prey availability may restrict spider populations below the level set by prey density, at times.
To judge from the microhabitats used by A. aurantia and by A. trifasciata it is reasonable to suppose that the former species depends primarily on insects living in the lowest stratum of herbage, and probably derived from the litter food chain, when immature. The immatures of A. trifasciata do seem to use more of the insect types which derive nourishment from the growing tips of the vegetation. Such a qualitative difference in use of foods might be significant for the ecological distinction between the resources used by the two species.
Forest ecosystems show a much greater increase in vertical distances than in number of vertical layers, from field-type habitats. Fitch (1963) found only four common araneid species to coexist in three layers (Elton 1966: field layer, low canopy, high canopy). Micrathene gracilis coexisits with the smaller M. mitrata in low canopy, eating both insects of the forest understory and those from other layers, which fly in that zone (in aerial pathways; Lubin 1973). M. sagitata lives in the field layer (or herb layer) according to Fitch (1963), Berry (1967) and my own observations. Verrucosa arenata, which Fitch considers syntopic with the first two Micrathene species, appears to live in the canopy of the understory or higher as an immature, possibly exploiting a qualitatively distinct food resource there, the insects derived from the canopy foliage. This and the first two Micrathene species as adults live in the same living space, across woodland paths, in which insects must fly (Lubin 1973). (Of course, the number of sympatric araneid species which can coexist might simply be doubled by including the nocturnal species which take down their webs by day, in addition to the four diurnal species described to use webs by day as well as night. Bike Argiope. For this doubling of species number, no more-complex behaviour of web-site selection would be required.)
Neoscona hentzi, Mecynogea lemniscata, Araneus cornutus, Mangora maculata and Micrathene gracilis tested in my screen-wire cages all built webs in the upper third of the cages when released at the bottom, in the unbagged condition. These araneid spiders all can occur in woodlands. What is difficult to understand, therefore, is how some forest species make the discrimination between the open spaces below the canopy and open spaces lower down, which, according to the logic outlined in the previous paragraph, is necessary for the coexistence of the common araneid species of forests. The choice by the spiders may be of different-size holes in which to place the web (or, the use of longer or shorter attachment threads from the body of the orb-web).
Is Web-site Selection Important to the Spider?
Above, I pointed to the fact that the webs of many adult A. aurantia are placed in a virtually horizontal plane; web-sites to construct the web in the preferred vertical orientation are invariably well within the distance that this species will travel in order to leave a woodland.
ENDERS: CHOICE OF WEB-SITE
709
Therefore, at least some of the A. aurantia do not move very far, nor seek very long, to find a web-site. In addition, the deviation of the height at which the web is placed, as a result of release at different heights, and the lack of any evident distress or discomfort movements made by the A. aurantia, even when these spiders had been released in extremely short vegetation where they could not build webs, both support this idea that selection of web-site is not a compelling choice which the spider must make.
I have watched many spiders off their webs (araneids, diguetids and theridiids, especially). The behaviour of such spiders, presumably or potentially seeking a site at which to build, may be described as hesitant, slow and lingering. A. aurantia walking about the vegetation at night make long pauses, up to several hours long. In contrast to the other behaviour of spiders (attack, copulation, and web-building), the choice of web-site occurs slowly, and considerable time is required before the maximal response is shown to the factors which have been proven to affect web-site selection. Web destruction and moulting show effects on website tenacity that last several days after treatment (Enders 1975a, 1976c). Also, several days are needed for the spider to correct the tendency to build near the point of release (either height, above, or habitat, Enders 1973). Responses delayed until after nightfall include the choices of humidity and light in laboratory gradients, and the start of sustained ‘searching’ for a web-site, and the start of web-building, after release in laboratory or in field. Similarly, the influence of moulting (Enders 1973), vegetation removal and web destruction (Enders 1976c) are not shown until nightfall, even though the treatments or moulting occurred during the daytime. Such hesitant behaviour was observed in the second instar spiderlings both in the laboratory and in the field when they emerged from egg sacs. Pauses similar to these of araneids off the web do occur in early stages of web building (phase 1, and also between phases 2 and 3; Peters 1970), and while the spider is waiting for prey, and before copulation, and during courtship behaviour. Observations of activity of Araneus diadematus by R. Ramousse & F. Davis (1977), using remote sensing (ultrasound), indicate that undisturbed araneids off the web normally show long pauses at one location.
These data suggest that choice of web-site may be subordinated to the avoidance of
predation, while the spider is off the web, and particularly in the daytime. Faster or more continuous activity would probably bring more costs of predation than benefits of better websites. Note that I observed spiders to move mainly when they were struck by puffs of wind; perhaps the movements are timed to coincide with movement of the vegetation, which would help avoid predation, both from visual and from tactile predators. I reported elsewhere (1974, 1975b) that likely predators are the visual salticid spiders (Tolbert 1975). Vertebrates also must take many spiders.
Comparison of Site Selection by Spiders with that of Other Animals.
The extent to which organisms actively choose a place to live varies considerably. Microorganisms, whose propagules are found almost everywhere (Beijerinck’s Law; Van der Pijl 1969), are selected by the environment. Animals with lower reproduction rates must more actively search for a suitable place to live, as in the case of vertebrates (Klopfer 1962). Web spiders seem more selective than microbes, less so than vertebrates. Levins (1968) mathematically describes that the behaviour of habitat selection must be the evolutionary result of the occurrence of different survival and reproduction rates among habitats. Innumerable factors can influence the evolution of habitat selection, factors which range from abiotic mortality factors to such biotic factors as competition and predation. Considering the multiplicity of such factors, one does not expect any really general unifying behavioural principles to underlie the way in which unrelated species select their particular habitats. Nonetheless, groups of a few species with similar habitats, similar feeding methods (‘guilds’, Root 1967; ‘functional groups’, Cummins 1974), or close taxonomic relation are expected to be similar in the way they select habitat either because they face similar physiological difficulties (Camin 1963), or because of adaptation to locate particular prey types (Enders 1977), or because of the accident of taxonomic relation. Below I attempt some inductive logic, comparing spider behaviour to that of other groups, rather than attempt here the deductive arguments of some of my other papers (1977, and in preparation).
The experimental investigations of vertebrates (Klopfer 1962; Wecker 1963; Sale 1968, 1969a, 1969b) have demonstrated that normal habitat can be selected on the basis of the shape of
710
ANIMAL BEHAVIOUR, 25, 3
physical space. This physiognomy is used as a token stimulus which stands for the important factors known to be of direct importance to the animal’s survival; responses are made not to the ultimate factor, but to some proximate factor which stands for the ultimate ‘goal’. Other authors have provided much non-experimental evidence that, in direct contrast to the free-ranging animal, the roost or nest used by vertebrates is influenced mainly by responses made directly to physical conditions and to the actual presence of predators and competitors (Daan & Wichers 1968; Cody 1969; Denny 1969; Wallin 1969; Grant 1971).
Herbivorous (and parasitoid) insect species clearly respond mainly to food cues, rather than to token, habitat cues, in most cases by chemical senses (Thorsteinson 1960; Dethier 19711 Schoonhoven 1972).
One detailed study of site selection by a predatory insect also involves odours of the chief prey of Thanasimus dubius, a clerid beetle (Vite & Williamson 1970). Ladybird beetles, less-specialized predators, seem to use light and humidity, token stimuli, to select vertical strata with suitable food (and microclimate) (Ewart & Chiang 1966).
Predatory lacewing insects seem to use both the odour of food (K. S. Hagen unpublished) and choice of height (Enders unpublished). Therefore, predatory insects seem to beBn-between the polyphagous spiders and the parasitoid and herbivorous insects in the way they choose a place to live, since the web spiders seem to mainly use token stimiil#
In contrast to terrestrial herbivorous and parasitoid arthropods, aquatic benthic invertebrates, even burrowing forms, seem to use token stimuli to choose a place to live (Keetch & Moran 1966; Hubschmann 1970; Jones 1970L though the review by Meadows & Campbell (1972) gave no synthetic conclusions.
Due to high web-site tenacity (Enders 1975a), an analogy to the roosts of vertebrates suggests itself. Like roost selection, the choice of web-site by araneid spiders (and habitat^ selection by sessile benthic invertebrates) should involve direct measurements by the animals of the factors known to be important for the survival of the species involved. But, the evidence so far (Duffey 1966; Eberhard 1971; Enders 1973, and this paper) indicates that the araneid spiders and perhaps the sessile aquatic invertebrates may more often use token stimuli to select their habitats. This use of token stimuli
is possible only because both free-ranging vertebrates and web spiders (and perhaps also sessile aquatic invertebrates) are polyphagous (Elton 1966). The use of token stimuli by araneid spiders, as discussed, must be related to the cost-to-benefit ratio of an ecological survey of possible habitats in time, energy and possibility of predation. Clearly, in the case of the vertebrates, the benefits of avoiding predation or thermoregulation are so great for a resting vertebrate that the roost is chosen on the basis of actual environmental needs; but the cost of time taken from feeding in the active time-budget vertebrate obliges it to use token stimuli to select a feeding site.
Acknowledgments This research was in part supported by N.S.F. Grant GB 6246 to P. N. Witt, and is a portion of a Ph.D. thesis carried out under his guidance at North Carolina State University. During writing, the author received support from N.S.F. Grant GB 27152 to W. F. Blair. H. W. Levi confirmed the identification of the araneid spider species mentioned here. A great many other people have given me help during the work reported here. My son, Hans, should perhaps be singled out for his help in the field. J. S. Rovner provided a critical reading of a late draft of the manuscript.
REFERENCES Almquist, S. 1973. Habitat selection by spiders on coastal sand dunes in Scania, Sweden. Entomol. Scand., 4, 134-154.
Amos, T. G. 1969. The reactions of Carpophilus spp. (Coleoptera: Nitidulidae) to humidity with
reference to gradient steepness. Anim. Behav., 17, 9-13.
Barnes, H. D., Crisp, J. & Powell, H. T. 1951. Observations on the orientation of some species of barnacles. J. Anim. Ecol., 20, 227-242.
Barnes, R. D. 1953. The ecological distribution of spiders in non-forest maritime communities at Beaufort, North Carolina. Ecol. Monogr., 23, 315-337. Benforado, J. & Kistler, K. H. 1973. Growth of the orb weaver, Araneus diadematus, and correlation with web measurements. Psyche, 80, 90-100.
Berry, J. W. 1967. The distributional ecology of spiders in the old-field succession of the Piedmont region of North Carolina. Ph.D. thesis, Duke University, Durham, North Carolina.
Bilsing, S. W. 1920. Quantitative studies in the food of spiders. Ohio J. Sci., 20, 215-260.
Buskirk, R. E. 1975. Aggressive display and orb defence in a colonial spider, Metabus gravidus. Anim. Behav., 23, 560-567.
Camin, J. H. 1963. Relations between host-finding behavior and life histories in ectoparasitic Acarina. Adv. Acarol., 1, 411-424.
ENDERS: CHOICE OF WEB-SITE
711
Cherrett, J. M. 1964. The distribution of spiders on the Moor House National Nature Reserve, Westmoreland. J. Anim. Eco I., 33, 27-48.
Cody, M. L. 1969. Convergent characteristics in sym-patric species: a possible relation to interspecific competition and aggression. Condor, 71, 223-239.
Colebourn, P. H. 1974. The influence of habitat structure on the distribution of Araneus diadematus Clerck. J. Anim. Ecol., 43, 401-410.
Cummins, K. W. 1974. Structure and function of stream ecosystems. Bioscience, 24, 631-641.
Daan, S. & Wichers, H. J. 1968. Habitat selection of bats hibernating in a limestone cave. Z. Sâugetierk. 33, 252-289.
Davidson, V. S. 1932. The effects of seasonal variability upon animal species in total populations in a deciduous forest succession. Ecol. Monogr., 2, 305-334.
Denny, J. V. 1969. The yellow-shafted Flicker (Colaptes auratus) on Nantucket Island, Mass. Bird-Banding, 40, 290-308.
Dethier, V. G. 1971, A surfeit of stimuli: a paucity of receptors. Am. Scient., 59, 706-715.
Dewar, M. J. S. 1975. Quantum organic chemistry. Science, N. Y., 187, 1037-1044.
Duffey, E. 1962. A population study of spiders in limestone grassland, the field-layer fauna. Oikos, 13, 15-34.
Duffey, E. 1966. Spider ecology and habitat structure (Arach: Araneae). Senck. Biol., 47, 45-49.
Eberhard, W. G. 1971. The ecology of the web of Uloborus diversus. (Araneae: Uloboridae).
Oecologia, 6, 328-342.
Elton, C. S. 1966. Patterns of Animal Communities. London : Methuen.
Enders, p§ 1972. Web site selection by the spider Argiope aurantia Lucas and other orb weaving spiders (Araneidae). Ph.D. thesis. North Carolina State University, Raleigh, North Carolina.
Enders, F. 1973. Selection of habitat by the spider Argiope aurantia Lucas (Araneidae). Am. Midi. Nat., 90, 47-55.,
Enders, F. 1974. Vertical stratification in orb-web spiders and a consideration of other methods of coexistence. Ecology, 55, 317-328.
Enders, F. 1975a. Change of web site in Argiope spiders (Araneidae). Am. Midi. Nat., 94, 484-490.
Enders, F. 1976c. Effects of prey capture, web destruction and habitat physiognomy on web-site tenacity of Argiope spiders (Araneidae). J. Arachnol., 3, 75-82.
Enders, F. 1975b. The influence of hunting manner on prey size, particularly in spiders with long attack distance (Fams. Araneidae, Linyphiidae and Salticidae). Am. Nat., 109, 732-763.
Enders, F. 1977. Possible biomechanical basis for guilds of insectivores. Southw. Nat., in press.
Enders, F. 1976a. Size, food-finding and Dyar’s constant: nymphal growth in spiders and coexistence of species in the Lycosidae. Environ. Entomol., 5, 1-10.
Enders, F. 1976b. Clutch size related to hunting manner of spider species. Ann. Entomol. Soc. Amer., 69, 991—998
Ewart, M. A. & Chiang, H. C. 1966. Effects of some environmental factors on the distribution of three species of Coccinellidae in their microhabitat. In : Symposium on the Ecology of Aphidophagous Insects (Èd. by I. Hodek), pp. 195-219.
Fitch, H. A. 1963. Spiders of the University of Kansas Natural History Reservation and Rockefeller Experimental Tract. Mise. Publ. Univ. Kans. Mus. Nat. Hist., 33, 1-202.
Golley, F. B. 1972. Summary. In: Papers from a Symposium on Tropical Ecology with an Emphasis on Organic Productivity (Ed. by F. B. Golley & V. S. Golley), pp. 407-413. Athens, Georgia: Institute of Ecology.
Grant, P. R. 1971. Interactive behavior of Puffins (Fratercula arctica L.) and Skuas (Stercorarius parasiticus L.). Behaviour, 40, 263-281.
Harwood, R. H. 1974. Predatory behavior of Argiope aurantia (Lucas), Am. Midi. Nat., 91, 130-139.
Holling, C. S. 1964. The analysis of complex population processes. Can. Ent., 96,335-347.
Hubschman, J. H. 1970. Substrate discrimination in Pectinatella magnifica Leidy (Bryozoa). J. Exp. Biol., 52,603-607.
Jones, D. A. 1970. Factors affecting the distribution of the intertidal isopods Eurydice pulchra Leach and E. affinis Hansen in Britain. J. Anim. Ecol., 39, 455-472.
Kaston, B. J. 1948. Spiders of Connecticut. State Geol. and Natural Hist. Surv. Conn. Bull., No. 70.
Keetch, D. P. & Moran, V. C. 1966. Observations on the biology of nymphs of Paragomphus cognatus (Rambur) (Odonata: Gomphidae). I. Habitat selection in relation to substrate particle size. Proc. Roy. Ent. Soc. Lond. Ser. A, 41, 116-122.
Kennedy, C. H. 1950. The relation of American dragon-fly-eating birds to their prey. Ecol. Mdnogr., 20, 103-142.
Klopfer, P. H. 1962. Behavioral Aspects of Ecology. Englewood Cliffs, N.J.: Prentice-Hall.
Levi, H. W. 1968. The spider genera Gea and Argiope in America (Araneae: Araneidae). Bull. Mus. Comp. Zool., 136, 319-353.
Levins, R. 1968. Evolution in Changing Environments: Some Theoretical Explorations. Princeton, N.J. : Princeton University Press.
Lowrie, D. C. 1948. The ecological succession of spiders of the Chicago area dunes. Ecology, 29, 334-351.
Lubin, Y. D. 1973. Web structure and function: the nonadhesive orb-web of Cyrtophora moluccensis (Doleschall) (Araneae: Araneidae). Forma et Functio, 6, 337-358.
Lubin, Y. D. 1974. Adaptive advantages and the evolution of colony formation in Cyrtophora (Araneae : Araneidae). Zool. J. Linn. Soc., 54, 321-339.
Luczak, J. 1963. Differences in the structure of communities of web spiders in one type of environment (young pine forest). Ekologia Polska, 11, 159-221.
Margalef, R. 1968. Perspectives in Ecological Theory. Chicago, 111.: University of Chicago Press.
McCook, H. C. 1889-1893. American Spiders and Their Spinning Work. 3 vols. Philadelphia, Pa.: Author and Acad. Nat. Sci. (Phila.).
Meadows, P. S. & Campbell, J. I. 1972. Habitat selection by aquatic invertebrates. Adv. Mar. Biol., 10, 271-382.
Muma, M. H. & Muma, K. E. 1949. Studies on a population of prairie spiders. Ecology, 30, 485-503.
Odum, E. P. 1959. Fundamentals of Ecology. 2nd Edn. Philadelphia, Pa.: Saunders.
712
ANIMAL BEHAVIOUR, 25, 3
Peters, P. J. 1970. Orb web construction: interaction of spider (Araneus diadematus Cl), and thread configuration. Anim. Behav., 18, 478-484.
Pianka, E. R. 1970. On r- and K- selection. Am. Nat., 104, 592-597.
Ramousse, R. and F. Davis III. 1976. Web-building time in a spider: preliminary applications of ultrasonic detection. Physiology & Behav., 17, 997-1000.
Reed, C F., Witt, P. N. & Scarboro, M. B. 1969. The orbweb during the life of Argiope aurantia (Lucas). Dev. Psychobiol., 2, 120-129.
Riechert, S. E. 1974. The pattern of local web distribution in a desert spider: mechanisms and seasonal variation. J. Anim. Ecol., 43, 733-746.
Riechert, S. E., Reeder, W. G. & Allen, T. 1973. Patterns of spider distribution (Agelenopsis aperta (Gertsch) in desert grassland and recent lava bed habitats, south-central New Mexico. J. Anim. Ecol., 42, 19-35.
Riechert, S. E. & Tracy, C. R. 1975. Thermal balance and prey availability: bases for a model relating web-site characteristics to spider reproductive success. Ecology, 56, 265-284.
Robinson, M. H. & Olazarri, J. 1971. Units of behavior and complex sequences in the predatory behavior of Argiope argentata (Fabricius) (Araneae: Araneidae). Smithson. Contr. Zool., 65, 1-36.
Robinson, M. H. & Robinson, B. 1970. Prey caught by a sample population of the spider Argiope argentata (Araneae: Araneidae) in Panama: a year’s census data. Zool. J. Linn. Soc., 49, 345-357.
Root, R. B. 1967. The niche exploitation pattern of the Blue-Gray Gnatcatcher. Ecol. Monogr., 37, 317-350.
Sale, P. F. 1968. Influence of cover availability on depth preference of the juvenile Manini, Acanthurus triostegus sandvicensis. Copeia, 1968, 802-807.
Sale, P. F. 1969a. A suggested mechanism for habitat selection by the juvenile Manini Acanthurus triostegus sandvicensis Streets. Behaviour, 35, 27-44.
Sale, P. F. 1969b. Pertinent stimuli for habitat selection by the juvenile Manini, Acanthurus triostegus sandvicensis. Ecology, 50, 616-623.
Savory, T. H. 1930. Environmental differences of spiders of the genus Zilla. J. Ecol., 18, 384-385.
Schaefer, M. 1970. Einfluss der Raumstruktur in Land-schaften der Meeres Kueste auf das Verteilungs-muster der Tierwelt. Zool. J. Syst., 95, 55-124.
Schoener, T. W. 1971. The theory of feeding strategies. Ann. Rev. Ecol. Syst., 2, 369-404.
Schoonhoven, L. M. 1972. Plant recognition by lepidop-terous larvae. Symp. Roy. Entomol. Soc. London, 6, 87-100.
Snedecor, G. W. & Cochran, W. G. 1967. Statistical Methods. Ames, Iowa: Iowa State University Press.
Thorsteinson, A. J. 1960. Host selection in phytophagous insects. Ann. Rev. Entomol., 5, 193-217.
Tilquin, A. 1942. La Toile Géométrique des Araignées. Paris: Presses Universitaires de France.
Tolbert, W. W. 1975. Predator avoidance behaviors and web defensive structures in the orb weavers Argiope aurantia and Argiope trifasciata (Araneae: Araneidae). Psyche, 82, 29-52.
Turnbull, A. L. 1973. Ecology of the true spiders (Araneomorphae). Ann. Rev. Entomol., 18, 305-348.
Van der Pijl, L. 1969. Principles of Dispersal in Higher Plants. Berlin: Springer-Verlag.
Vite, J. P. & Williamson, D. L. 1970. Thanasimus dubius: Prey perception. J. Insect Physiol., 16, 233-239.
Wallin, L. 1969. The Japanese bat fauna. Zool. Bidrag. Fran Uppsala, 37, 223-440.
Wecker, S. C. 1963. The role of early experience in habitat selection by the prairie deer mouse, Peromyscus maniculatus bairdi. Ecol. Monogr., 33, 307-325.
Wellington, W. G. 1965. Some maternal influences on progeny quality in the western tent caterpillar Malacosoma pluviale (Dyar). Can. Ent., 97, 1-14.
Wilder, B. G. 1873. The habits and parasites of Epeira (Argiope) riparia, with a note on the moulting of Nephila plumipes. Proc. Am. Ass. Adv. Sci., 22, 257-263.
Winston, P. W. & Bates, D. H. 1960. Saturated solutions for the control of humidity in biological research. Ecology, 41, 232-237.
Witt, P. N., Reed, C. F. & Peakall, D. B. 1968. A Spider’s Web. Berlin: Springer-Verlag.
(Received 20 January 1975; revised 25 November 1975: MS. number: a1661)